74 gesichtete, geschützte Fragmente: Plagiat
| [1.] Mrs/Fragment 001 04 - Diskussion Bearbeitet: 17. April 2015, 20:14 WiseWoman Erstellt: 27. December 2014, 11:31 (Klgn) | BauernOpfer, Fragment, Gesichtet, Mrs, Poole and Owens 2003, SMWFragment, Schutzlevel sysop |
|
|
| Untersuchte Arbeit: Seite: 1, Zeilen: 4-12 |
Quelle: Poole and Owens 2003 Seite(n): XI, Zeilen: 2-5, 12-16 |
|---|---|
| In recent years, nanotechnology has become one of the most important and exciting forefront fields in Physics, Chemistry, Engineering and Biology. It shows great promise for providing us in the near future with many breakthroughs that will change the direction of technological advances in a wide range of applications.
The current widespread interest in nanotechnology dates back to the years 1996 to 1998 when a panel under the auspices of the World Technology Evaluation Center (WTEC), funded by the National Science Foundation and other federal agencies, undertook a worldwide study of research and development in the area of nanotechnology, with the purpose of assessing its potential for technological innovation [1]. [1] C. P. Poole, Jr., Frank J. Owens, Introduction to nanotechnology, John Wilez & Sons,Inc., Hoboken, New Jersey (2003) |
In recent years nanotechnology has become one of the most important and exciting forefront fields in Physics, Chemistry, Engineering and Biology. It shows great promise for providing us in the near future with many breakthroughs that will change the direction of technological advances in a wide range of applications. [...]
The current widespread interest in nanotechnology dates back to the years 1996 to 1998 when a panel under the auspices of the World Technology Evaluation Center (WTEC), funded by the National Science Foundation and other federal agencies, undertook a world-wide study of research and development in the area of nanotechnology, with the purpose of assessing its potential for technological innovation. |
_ |
|
| [2.] Mrs/Fragment 001 13 - Diskussion Bearbeitet: 25. January 2015, 12:36 WiseWoman Erstellt: 27. December 2014, 14:30 (SleepyHollow02) | Fragment, Gesichtet, Monserrat De La Luz Garcia Curiel 2004, Mrs, SMWFragment, Schutzlevel sysop, Verschleierung |
|
|
| Untersuchte Arbeit: Seite: 1, Zeilen: 13-29 |
Quelle: Monserrat De La Luz Garcia Curiel 2004 Seite(n): 1, Zeilen: 4 ff. |
|---|---|
| However, it is during the past decade that nanotechnology went through a variety of disciplines. From chemistry to biology, from materials science to electrical engineering, scientists are creating the tools and developing the expertise to bring nanotechnology out of the research labs and into the market place. Nanocomposite materials, when using organic polymer and inorganic fillers, represent a merger between traditional organic and inorganic materials, resulting in compositions that are truly hybrid. Nature has created many (composite) materials, such as diatoms, radiolarian and bone [2], from which scientists can learn. Organic-inorganic composites with nanoscale dimensions are of growing interest because of their unique properties, and numerous potential applications such as enhancement of conductivity, toughness , optical activity [3], catalytic activity [4], chemical selectivity [5,6] etc. In these materials, inorganic and organic components are mixed or hybridized at nanometer scale with virtually any composition leading to the formation of hybrid/nanocomposite materials. Ceramics are generally known for their hardness and brittleness, along with their resistance to high temperatures and severe physical/chemical environments. In addition, many inorganic materials such as silica glass have excellent optical properties such as transparency [7]. For most applications, the brittleness (lack of impact strength) is the major, sometimes fatal, deficiency of ceramics. On the other hand, organic polymers are usually noted for their low density and high toughness. (i.e. high impact [strength), However, lack of hardness is one of the most significant flaws of polymers in many applications.]
[2] D.B. Porter, Conference Proceedings from Organic-Inorganic hybrids conference Guildford, U.K. June (2000). [3] J.G. Winiarz, L.M. Zhang, M. Lal, Friend, Journal of the American Chemical Society, 121, 5287 (1999). [4] S.N. Sidorov, et al. Journal of the American Chemical Society, 123, 10502 (2001). [5] T.C. Merkel, B.D. Freeman, R.J. Spontak, American Journal of Science 296, 519 (2002). [6] C. Joly, M. Smaihi, L. Porcar, Chemistry of Materials, 11, 2331 (1999). [7] G. Wypych, Handbook of fillers 2nd Ed. New York (1999). |
However, it is during the past decade that nanotechnology went through a variety of disciplines. From chemistry to biology, from materials science to electrical engineering, scientists are creating the tools and developing the expertise to bring nanotechnology out of the research labs and into the market place. Nanostructured composite materials, when using organic polymer and inorganic fillers, represent a merger between traditional organic and inorganic materials, resulting in compositions that are truly hybrid. Nature has created many (composite) materials, such as diatoms, radiolarian [2] and bone [3], from which scientists can learn (Fig. 1). Organic-inorganic composites with nanoscale dimensions are of growing interest because of their unique properties, and numerous potential applications such as enhancement of conductivity [4,5], toughness [6], optical activity [7,8], catalytic activity [9], chemical selectivity [10,11] etc. In these materials, inorganic and organic components are mixed or hybridised at nanometer scale with virtually any composition leading to the formation of hybrid/nanocomposite materials [12-22]. [...].
Ceramics are generally known for their hardness and brittleness, along with their resistance to high temperatures and severe physical/chemical environments [23, 24]. In addition, many inorganic materials such as silica glass have excellent optical properties such as transparency [25]. For most applications, the brittleness (lack of impact strength) is the major, sometimes fatal, deficiency of ceramics [23]. On the other hand, organic polymers are usually noted for their low density and high toughness. (i.e., high impact strength). [2] Volkmer, D. Chemie in unserer Zeit 33, 6 (1999). [3] Porter, D.B. Conference Proceedings from Organic-Inorganic hybrids conference Guildford, U.K. June (2000). [4] Coronado, E., Galan-Mascaros, J.R., Gomez-Garcia, C.J. and Laukhin, V., Nature 408, 447 (2000). [5] Croce, F., Appetecchi, G.B., Persi, L. and Scrosati, B. Nature 394, 456 (1998). [6] Pinnavaia, T.J. Science 220, 365 (1983). [7] Wang, Y. and Herron, N. Science 273, 632 (1996). [8] Winiarz, J.G., Zhang, L.M., Lal, M., Friend, C.S. and Prasad, P.N. J. Am. Chem. Soc. 121, 5287 (1999). [9] Sidorov, S.N. et al. J. Am. Chem. Soc. 123, 10502 (2001). [10] Merkel, T.C. Freeman, B.D., Spontak, R.J., He, Z., Pinnau, I., Meakin, P. and Hill, A.J. Science 296, 519 (2002). [11] Joly, C., Smaihi, M., Porcar L. and Noble, R.D. Chem. Mater. 11, 2331 (1999). [12] Hajji, P., David, L., Gerard, J.F., Pascault, J.P. and Vigier, G. J. Polym. Sci. 37, 3172 (1999). [13] Sanchez, C., Ribot, F. and Lebeau, B. J. Mater. Chem. 9, 35 (1999). Sanchez, C., LeBeau, B.and Ribot, F. J. Sol-Gel Sci. Tech 19, 31 (2000). [14] Pomogailo, A. D. Russ. Chem. Rev. 69, 53 (2000). [15] Hajji, P., David, L., Gerard, J.F, Kaddami, H., Pascault, J.P. and Vigier, G. Mater. Res. Symp. Proc. 576, 357 (1999). [16] Novak, B.M. Adv. Mater. 5, 422 (1993). [17] Lichtenha, J.D., Schwab, J.J. and Reinerth, W.A. Chem. Innovation 31, 3 (2001). [18] Sanchez, C. and Ribot, F. New J. Chem. 18, 1007 (1994). [19] Ellsworth, M.W. and Gin, D.L. Polymer News 24, 331 (1999). [20] Kwiatkowski, K. C. and Lukehart, C. M. in Handbook of Nanostructured Materials and Nanotechnology, Volume 1: Synthesis and Processing, Nalwa, H. S. Ed. Academic Press, San Diego, CA (2000). [21] Schubert, U., Hüsing, N. and Lorenz, A. Chem. Mater. 7, 2010 (1995). [22] Morikawa, A., Iyoku, Y., Kakimoto, M. and Imai, Y. J. Mater. Chem. 2, 679 (1992). [23] Reed, J.S. Principles of Ceramics Processing 2nd Ed. (1995). [24] Richerson, D.W. Modern Ceramic Engineering 2nd Ed. (1992). [25] Wypych G. Handbook of fillers 2nd Ed. New York (1999). |
No source is given. |
|
| [3.] Mrs/Fragment 002 01 - Diskussion Bearbeitet: 25. January 2015, 14:09 WiseWoman Erstellt: 27. December 2014, 15:17 (Klgn) | Fragment, Gesichtet, Monserrat De La Luz Garcia Curiel 2004, Mrs, SMWFragment, Schutzlevel sysop, Verschleierung |
|
|
| Untersuchte Arbeit: Seite: 2, Zeilen: 1-9 |
Quelle: Monserrat De La Luz Garcia Curiel 2004 Seite(n): 1 f., Zeilen: 1: last line, 2: 1 ff. |
|---|---|
| However, lack of hardness is one of the most significant flaws of polymers in many applications. Associated with the lack of hardness are the problems of low wear and scratch resistance as well as dimensional stability. The developments of conventional composite materials with ceramics as fillers and polymers as matrices are being researched extensively. Important examples of these composite materials are the semi-crystalline polymers mixed with inorganic particles. They consist of an amorphous-crystalline matrix (with a lamella thickness of typical size of 10 to 100 nm) and dispersed nanoparticles. They can be tailor-made to exhibit excellent elasticity (e.g., synthetic rubber) or optical transparency (e.g., polymethacrylates or Plexiglas). | They can
[page 2] be tailor-made to exhibit excellent elasticity (e.g., synthetic rubber) or optical transparency (e.g., polymethacrylates or PlexiglasTM). However, lack of hardness is one of the most significant flaws of polymers in many applications. Associated with the lack of hardness are the problems of low wear and scratch resistance as well as dimensional stability [26]. The developments of conventional composite materials with ceramics as fillers and polymers as matrices are being researched extensively. Important examples of these composite materials are the semi-crystalline polymers mixed with inorganic particles [27]. They consist of an amorphous-crystalline matrix (with a lamella thickness of typical size of 10 to 100 nm) and dispersed nanoparticles. [26] Hutchings, I.M. Tribology. Friction and wear of engineering materials. Ed. Edward Arnold (1992). [27] Schrauwen, B. Deformation and failure of semi-crystalline polymer systems. PhD Thesis University of Eindhoven. The Netherlands. (2003). |
No source is given. |
|
| [4.] Mrs/Fragment 002 13 - Diskussion Bearbeitet: 25. April 2015, 22:33 WiseWoman Erstellt: 24. January 2015, 16:25 (Klgn) | BauernOpfer, Bhattacharya et al. 2008, Fragment, Gesichtet, Mrs, SMWFragment, Schutzlevel sysop |
|
|
| Untersuchte Arbeit: Seite: 2, Zeilen: 13-20 |
Quelle: Bhattacharya et al. 2008 Seite(n): 1, Zeilen: 2-9 |
|---|---|
| Nanotechnology has created a key revolution in the 21th century exploiting the new properties, phenomena and functionalities exhibited by matters when dealt at the level of few nanometers as opposed to hundred nanometers and above [8]. Nanoscale materials are already recognized as unique because they produce qualitatively new behavior when compared with their macroscopic counterpart. It is understood that when the domain size within the materials becomes comparable with the physical length scale, such as segments of a polymer macromolecule, the expected physical phenomena and the response to any external disturbance do not follow the established principles.
[8] S. N.Bhattacharya, Rahul K. Gupta, Polymeric Nanocomposites Theory and Practice, Hanser 2008 |
Nanotechnology has created a key revolution in the 21st century exploiting the new properties, phenomena and functionalities exhibited by matters when dealt at the level of few nanometers as opposed to hundred nanometers and above. Nanoscale materials are already recognized as unique because they produce qualitatively new behavior when compared with their macroscopic counterparts. It is understood that when the domain size within the materials becomes comparable with the physical length scale, such as segments of a polymer macromolecule, the expected physical phenomena and the response to any external disturbance do not follow the established principles. |
|
|
| [5.] Mrs/Fragment 002 20 - Diskussion Bearbeitet: 24. January 2015, 23:23 WiseWoman Erstellt: 28. December 2014, 16:17 (Graf Isolan) | BauernOpfer, Fragment, Gesichtet, Mrs, Paul and Robeson 2008, SMWFragment, Schutzlevel sysop |
|
|
| Untersuchte Arbeit: Seite: 2, Zeilen: 20-33 |
Quelle: Paul and Robeson 2008 Seite(n): 3187, Zeilen: li. Spalte 2ff. - re. Sp. 1 |
|---|---|
| The field of nanotechnology is one of the most popular areas for current research and development in all technical disciplines. This obviously includes polymer science and technology and even in this field, the investigations cover a broad range of topics. This would include microelectronics (which could now be referred to as nanoelectronics) as the critical dimension scale for modern devices is now below 100 nm. Other areas include polymer-based biomaterials, nanoparticle drug delivery, mini emulsion particles, fuel cell electrode polymer bound catalysts, layer-by-layer self-assembled polymer films, electrospun nanofibers, imprint lithography, polymer blends and nanocomposites. Even in the field of nanocomposites, many diverse topics exist including composite reinforcement, barrier properties, flame resistance, electro-optical properties, cosmetic applications, bactericidal properties. Nanotechnology is not new to polymer science as prior studies before the age of nanotechnology involved nanoscale dimensions but were not specifically referred to as nanotechnology until recently. Phase separated polymer blends often achieve nanoscale phase dimensions; block copolymer domain morphology is usually at the nanoscale level; asymmetric [membranes often have nanoscale void structure, mini emulsion particles are below 100 nm; and interfacial phenomena in blends and composites involve nanoscale dimensions.] | 1. Introduction
The field of nanotechnology is one of the most popular areas for current research and development in basically all technical disciplines. This obviously includes polymer science and technology and even in this field the investigations cover a broad range of topics. This would include microelectronics (which could now be referred to as nanoelectronics) as the critical dimension scale for modern devices is now below 100 nm. Other areas include polymer-based biomaterials, nanoparticle drug delivery, miniemulsion particles, fuel cell electrode polymer bound catalysts, layer-by-layer self-assembled polymer films, electrospun nanofibers, imprint lithography, polymer blends and nanocomposites. Even in the field of nanocomposites, many diverse topics exist including composite reinforcement, barrier properties, flame resistance, electro-optical properties, cosmetic applications, bactericidal properties. Nanotechnology is not new to polymer science as prior studies before the age of nanotechnology involved nanoscale dimensions but were not specifically referred to as nanotechnology until recently. Phase separated polymer blends often achieve nanoscale phase dimensions; block copolymer domain morphology is usually at the nanoscale level; asymmetric membranes often have nanoscale void structure, miniemulsion particles are below 100 nm; and interfacial phenomena in blends and composites involve nanoscale dimensions. |
A reference to the source is found on the next page, but the almost identical wording is not made clear. |
|
| [6.] Mrs/Fragment 003 01 - Diskussion Bearbeitet: 24. January 2015, 23:30 WiseWoman Erstellt: 28. December 2014, 16:31 (Graf Isolan) | BauernOpfer, Fragment, Gesichtet, Mrs, Paul and Robeson 2008, SMWFragment, Schutzlevel sysop |
|
|
| Untersuchte Arbeit: Seite: 3, Zeilen: 1-12 |
Quelle: Paul and Robeson 2008 Seite(n): 3187, Zeilen: li. Spalte 19-23 - re. Sp. 1 |
|---|---|
| [Phase separated polymer blends often achieve nanoscale phase dimensions; block copolymer domain morphology is usually at the nanoscale level; asymmetric] membranes often have nanoscale void structure, mini emulsion particles are below 100 nm; and interfacial phenomena in blends and composites involve nanoscale dimensions. Even with nanocomposites, carbon black reinforcement of elastomers, colloidal silica modification and even naturally occurring fiber (e.g., asbestos-nanoscale fiber diameter) reinforcement are subjects that have been investigated for decades [9].
Almost lost in the present nanocomposite discussions are the organic–inorganic nanocomposites based on sol–gel chemistry which have been investigated for several decades [10]. In essence, the nanoscale of dimensions is the transition zone between the macro level and the molecular level. Recent interest in polymer matrix based nanocomposites has emerged initially with interesting observations involving exfoliated clay and more recent studies with carbon nanotubes, carbon nanofibers, exfoliated graphite (graphene), nanocrystalline metals and a host of additional nanoscale inorganic filler or fiber modifications. [9] D.R. Paul, L.M. Robeson, Polymer 49 (2008) 3187–3204 [10] J.E.Mark, CY. Jiang, MY. Tang, Macromolecules 1984; 17:2613–6. |
Phase separated polymer blends often achieve nanoscale phase dimensions; block copolymer domain morphology is usually at the nanoscale level; asymmetric membranes often have nanoscale void structure, miniemulsion particles are below 100 nm; and interfacial phenomena in blends and composites involve nanoscale dimensions. Even with nanocomposites, carbon black reinforcement of elastomers, colloidal silica modification and even naturally occurring fiber (e.g., asbestos-nanoscale fiber diameter) reinforcement are subjects that have been investigated for decades. Almost lost in the present nanocomposite discussions are the organic–inorganic nanocomposites based on sol–gel chemistry which have been investigated for several decades [1-3]. In essence, the nanoscale of dimensions is the transition zone between the macrolevel and the molecular level. Recent interest in polymer matrix based nanocomposites has emerged initially with interesting observations involving exfoliated clay and more recent studies with carbon nanotubes, carbon nanofibers, exfoliated graphite (graphene), nanocrystalline metals and a host of additional nanoscale inorganic filler or fiber modifications.
[1] Mark JE, Jiang CY, Tang MY. Macromolecules 1984;17:2613–6. [2] Wilkes GL, Orler B, Huang H. Polym Prep 1985;26:300–1. [3] Wen J, Wilkes GL. Chem Mater 1996;8:1667–81. |
A reference to the original article is given once in the middle of the text. The word-for-word copy that is found both before and after this reference is not marked. There are slight formatting differences between the HTML version of the original article and the PDF. |
|
| [7.] Mrs/Fragment 003 24 - Diskussion Bearbeitet: 16. May 2015, 10:39 Hindemith Erstellt: 27. December 2014, 19:12 (SleepyHollow02) | Fragment, Gesichtet, KomplettPlagiat, Monserrat De La Luz Garcia Curiel 2004, Mrs, SMWFragment, Schutzlevel sysop |
|
|
| Untersuchte Arbeit: Seite: 3, Zeilen: 24-31 |
Quelle: Monserrat De La Luz Garcia Curiel 2004 Seite(n): 2, Zeilen: 20 ff. |
|---|---|
| ‘What are the potential uses of nanotechnology?’ In the limited number of years that nanotechnology has been investigated, a plethora of answers to this question have been presented. It seems that nanotechnology could potentially solve almost any problem; thus, a more interesting question is, 'what real problems will nanotechnology solve?' Nanocomposite technology has been described as the next great frontier of material science. For example, polymer resins containing well-dispersed layered silicate nanoclays are emerging as a new class of nanocomposites. The reason is that by employing minimal addition levels of filler (< 10 wt %) nanoclays enhance mechanical, thermal, dimensional and barrier performance properties [significantly.] | ‘What are the potential uses of nanotechnology?’ In the limited number of years that nanotechnology has been investigated, a plethora of answers to this question have been presented. It seems that nanotechnology could potentially solve almost any problem; thus, a more interesting question is, 'what real problems will nanotechnology solve?' Nanocomposite technology has been described as the next great frontier of material science. For example, polymer resins containing well-dispersed layered silicate nanoclays are emerging as a new class of nanocomposites. The reason is that by employing minimal addition levels of filler (< 10 wt%) nanoclays enhance mechanical, thermal, dimensional and barrier performance properties significantly. |
The source is not mentioned anywhere. |
|
| [8.] Mrs/Fragment 004 01 - Diskussion Bearbeitet: 16. May 2015, 10:43 Hindemith Erstellt: 27. December 2014, 20:10 (SleepyHollow02) | Fragment, Gesichtet, Monserrat De La Luz Garcia Curiel 2004, Mrs, SMWFragment, Schutzlevel sysop, Verschleierung |
|
|
| Untersuchte Arbeit: Seite: 4, Zeilen: 1-2 |
Quelle: Monserrat De La Luz Garcia Curiel 2004 Seite(n): 2, Zeilen: 30 ff. |
|---|---|
| It has been said that for every 1 wt% addition, a property increase on the order of 10% (or more) is realized. This loading-to-performance ratio is known as the “nano-effect”. | It has been said that for every 1 wt% addition, a property increase on the order of 10% (or more) is realized. This loading-to-performance ratio is known as the “nano-effect” [28].
[28] Hirschinger, J., Miura, H., Gardner, K.H. and English, A.D. Macromolecules 23, 2153 (1990). |
The passage starts on the previous page: Mrs/Fragment 003 24 The source is not mentioned anywhere, and the reference to Hirschinger et al. has been removed. |
|
| [9.] Mrs/Fragment 004 03 - Diskussion Bearbeitet: 25. April 2015, 22:34 WiseWoman Erstellt: 24. January 2015, 16:38 (Klgn) | BauernOpfer, Bhattacharya et al. 2008, Fragment, Gesichtet, Mrs, SMWFragment, Schutzlevel sysop |
|
|
| Untersuchte Arbeit: Seite: 4, Zeilen: 3-12 |
Quelle: Bhattacharya et al. 2008 Seite(n): 1, Zeilen: 17-30 |
|---|---|
| 1.3. Polymer Nanocomposite
Nanocomposite technology is a new developing field, in which nanofiller are added to a polymer to reinforce and provide novel characteristics. Nanocomposite technology is applicable to a wide range of polymers from thermoplastics and thermosets to elastomers. Two decades ago, researchers from Toyota Central Research and Development produced a new group of polymer-clay complexes or composites, which was aptly called polymer-layered silicate nanocomposite. Today, there is a variety of nanofillers used in nanocomposites. The most common types of fillers are natural clays, synthetic clays, nanostructured silicas, nanoceramics and carbon nanotubes. The property enhancements have allowed these materials to commercially compete with traditional materials [8]. [8] S. N.Bhattacharya, Rahul K. Gupta, Polymeric Nanocomposites Theory and Practice, Hanser 2008 |
1.1 Polymer Nanocomposites
Nanocomposite technology is a newly developed field, in which nanofillers are added to a polymer to reinforce and provide novel characteristics. Nanocomposite technology is applicable to a wide range of polymers from thermoplastics and thermosets to elastomers. Two decades ago, researchers from Toyota Central Research and Development produced a new group of polymer-clay complexes or composites, which was aptly called polymer-layered silicate nanocomposites or polymer nanocomposites. Today, there is a variety of nanofillers used in nanocomposites. Cost and availability continue to change as the field is relatively new and several of these fillers are still being developed. The most common types of fillers are natural clays (mined, refined and treated), synthetic clays, nanostructured silicas, nanoceramics, nanocalcium carbonates and nanotubes (carbon based). The properties conferred by the nanoparticles to the polymer matrix are remarkable. The property enhancements have allowed these materials to commercially compete with traditional materials. |
|
|
| [10.] Mrs/Fragment 009 04 - Diskussion Bearbeitet: 16. May 2015, 19:15 Hindemith Erstellt: 16. May 2015, 15:31 (Hindemith) | Cuentas 2003, Fragment, Gesichtet, Mrs, SMWFragment, Schutzlevel sysop, Verschleierung |
|
|
| Untersuchte Arbeit: Seite: 9, Zeilen: 4-6, 16-19 |
Quelle: Cuentas 2003 Seite(n): 2, Zeilen: 2 ff. |
|---|---|
| Organic-inorganic hybrid materials represent the natural interface between two worlds of chemistry (organic and inorganic) each with very significant contributions to the field of material science, and each with characteristic properties that result in diverse advantages and limitation. [...] Consequently the main idea when developing hybrid materials is to take advantage of the best properties of each component that forms the hybrid, trying to decrease or eliminate their drawbacks getting in an ideal way a synergic effect; which results in the development of new materials with new properties. | Organic-inorganic hybrid materials represent the natural interface between two worlds of chemistry (organic and inorganic) each with very significant contributions to the field of materials science, and each with characteristic properties that result in diverse advantages and limitations. The main idea when developing hybrid materials is to take advantage of the best properties of each component that forms the hybrid, trying to decrease or eliminate their drawbacks getting in an ideal way a synergic effect; which results in the development of new materials with new properties. |
The source is not mentioned. An alternative source could be: [1] |
|
| [11.] Mrs/Fragment 010 03 - Diskussion Bearbeitet: 25. April 2015, 22:37 WiseWoman Erstellt: 24. January 2015, 17:04 (Klgn) | Bhattacharya et al. 2008, Fragment, Gesichtet, Mrs, SMWFragment, Schutzlevel sysop, Verschleierung |
|
|
| Untersuchte Arbeit: Seite: 10, Zeilen: 3-13 |
Quelle: Bhattacharya et al. 2008 Seite(n): 22; 23, Zeilen: 22: 13-18 ; 23:1-5 |
|---|---|
| The large variety of polymer systems used in nanocomposite preparation can be classified as follow:
1. Thermoplastics 2.2.1. Thermoplastic Thermoplastics, such as polypropylene (PP), polyethylene (PE), copolymers such as poly (ethylene-co vinyl acetate) (EVA), poly (ethylene propylene diene) rubber (EPDM), polyamides (PA), poly – ethylene terephthalate (PET) and polystyrene (PS) have been used as polymer matrices for the preparation of nanocomposites. |
The large variety of polymer systems used in nanocomposite preparation can be conventionally classified as follows:
1. Thermoplastics [page 23] 2.3.1 Thermoplastics Thermoplastics, such as polypropylene (PP), polyethylene (PE), copolymers, such as poly (ethylene-co vinyl acetate) (EVA), polyethylene propylene diene) rubber (EPDM), polyamides (PA), poly-thylene terephtalate (PET), polystyrene (PST) and poly (1-butene) have been used as polymer matrices for the preparation of nanocomposites. |
|
|
| [12.] Mrs/Fragment 010 17 - Diskussion Bearbeitet: 16. May 2015, 13:22 Hindemith Erstellt: 29. March 2015, 06:58 (SleepyHollow02) | BauernOpfer, Fragment, Gesichtet, Lu et al 2006, Mrs, SMWFragment, Schutzlevel sysop |
|
|
| Untersuchte Arbeit: Seite: 10, Zeilen: 17-25 |
Quelle: Lu et al 2006 Seite(n): 485, Zeilen: abstract |
|---|---|
| HUIMIN LU et al. [25] prepared successfully PA66/nano- SiO2 composites by melt compounding technique. They studied the effect of nano- SiO2 on the morphology, crystallization and dynamic mechanical properties of polyamide 66. The influence of nano- SiO2 on the tensile fracture morphology of the nanocomposites was studied by scanning electron microscopy (SEM), which suggested that the nanocomposites revealed an extensive plastic elongation of the matrix polymer. The crystallization behavior of polyamide 66 and its nanocomposites were studied by differential scanning calorimetry (DSC). DSC nonisothermal curves showed an increase in the crystallization temperature along with increasing degree of crystallinity. Dynamic mechanical properties (DMA) indicated significant improvement in the storage modulus and loss modulus compared with neat polyamide 66.
[25] H. LU, X. XU, X. LI and Z. ZHANG, Bulletin of Materials Science, Vol. 29, No. 5, October 2006, pp. 485–490 |
This article addresses the effect of nano-SiO2 on the morphology, crystallization and dynamic mechanical properties of polyamide 66. The influence of nano-SiO2 on the tensile fracture morphology of the nanocomposites was studied by scanning electron microscopy (SEM), which suggested that the nanocomposites revealed an extensive plastic stretch of the matrix polymer. The crystallization behaviour of polyamide 66 and its nanocomposites were studied by differential scanning calorimetry (DSC). DSC nonisothermal curves showed an increase in the crystallization temperature along with increasing degree of crystallinity. Dynamic mechanical properties (DMA) indicated significant improvement in the storage modulus and loss modulus compared with neat polyamide 66. |
The source is given, but it is not clear that the description of the findings of the paper is taken verbatim from the abstract of the paper. The reader would expect an evaluation of the author of the thesis studied. |
|
| [13.] Mrs/Fragment 011 21 - Diskussion Bearbeitet: 16. May 2015, 20:07 WiseWoman Erstellt: 16. May 2015, 15:59 (Hindemith) | Ahn et al 2004, BauernOpfer, Fragment, Gesichtet, Mrs, SMWFragment, Schutzlevel sysop |
|
|
| Untersuchte Arbeit: Seite: 11, Zeilen: 21-32 |
Quelle: Ahn et al 2004 Seite(n): 812, Zeilen: abstract, first lines of introduction |
|---|---|
| High performance poly (ethylene 2, 6-naphthalate) (PEN), with its superior physical and mechanical properties, has been used in specialty films, fibers, and in blow moldings. However, the applications of PEN are limited, because PEN exhibits a relatively high melt viscosity, which makes fiber spinning and injection molding difficult. The effect of stearic acid modification on the dispersion quality of silica nanoparticles and the adhesion between the filler and polymer matrix with stearic acid concentration were investigated by Seon Hoon Ahn and co-workers [59]. Thus, the wettability of silica nanoparticles was improved by the addition of stearic acid. The presence of adsorbed stearic acid on the surface of the silica nanoparticles reduced the interaction between silica nanoparticles, and reduced as well the size of agglomerates with increasing concentration. Silica nanoparticle–reinforced poly (ethylene 2, 6-naphthalate) (PEN) composites were melt-blended to investigate their mechanical properties and the processability of the composites.
[59] Seon Hoon Ahn, Seong Hun Kim, Seung Goo Lee, Journal of Applied Polymer Science, Vol. 94, 812–818 (2004) PEN [sic] |
The effect of stearic acid modification on the dispersity of silica nanoparticles and the adhesion between the filler and polymer matrix with stearic acid concentration were investigated. The wettability of silica nanoparticles was improved by the addition of stearic acid. The presence of adsorbed stearic acid on the surface of the silica nanoparticles reduced the interaction between silica nanoparticles, and reduced the size of agglomerates with increasing concentration. Silica nanoparticle–reinforced poly(ethylene 2,6- naphthalate) (PEN) composites were melt-blended to investigate their mechanical properties and the processability of the composites. [...]
INTRODUCTION High-performance poly(ethylene 2,6-naphthalate) (PEN), with its superior physical and mechanical properties, has been used in specialty films, fibers, and in blow moldings.1–3 However, the applications of PEN are limited because PEN exhibits a relatively high melt viscosity, which makes fiber spinning and injection molding difficult.4 1. Kim, S. H.; Kang, S. W.; Park, J. K.; Park, Y. H. J Appl Polym Sci 1998, 70, 1065. 2. Kim, S. H.; Kang, S. W. Fibers Polym 2000, 1, 83. 3. Kim, J. Y.; Seo, E. S.; Kim, S. H.; Kikutani, T. Macromol Res 2003, 11, 62. 4. Ulcer, Y.; Cakmak, M. Polymer 1994, 35, 5651. |
The source is mentioned, but the reference only covers the text before the reference and it does not make clear that the text has been taken almost verbatim. |
|
| [14.] Mrs/Fragment 012 01 - Diskussion Bearbeitet: 16. May 2015, 20:09 WiseWoman Erstellt: 16. May 2015, 16:03 (Hindemith) | Ahn et al 2004, BauernOpfer, Fragment, Gesichtet, Mrs, SMWFragment, Schutzlevel sysop |
|
|
| Untersuchte Arbeit: Seite: 12, Zeilen: 1-3 |
Quelle: Ahn et al 2004 Seite(n): 812, Zeilen: abstract |
|---|---|
| [The torque and total torque values of the composites decreased with increasing] silica nanoparticle content. The tensile modulus of the composites reinforced with unmodified silica nanoparticles increased with increasing silica content, whereas the tensile strength and elongation decreased. | The torque and total torque values of the composites decreased with increasing silica nanoparticle content. The tensile moduli of the composites reinforced with unmodified silica nanoparticles increased with increasing silica content, whereas the tensile strength and elongation decreased. |
The source is mentioned on the previous page and it is clear that the results of the mentioned authors are described. It is not clear, however, that the abstract of the source has been copied verbatim. The passage starts on the previous page: Mrs/Fragment 011 21 |
|
| [15.] Mrs/Fragment 012 05 - Diskussion Bearbeitet: 19. April 2015, 19:06 WiseWoman Erstellt: 28. December 2014, 02:04 (Hindemith) | BauernOpfer, Fragment, Gesichtet, Mrs, SMWFragment, Schutzlevel sysop, Sertchook et al 2007 |
|
|
| Untersuchte Arbeit: Seite: 12, Zeilen: 5-21 |
Quelle: Sertchook et al 2007 Seite(n): 98, Zeilen: abstract |
|---|---|
| Polyethylene (PE) and silica are perhaps the simplest and most common organic and inorganic polymers, respectively. Sertchook et al. [27] describe, for the first time, a physically interpenetrating nanocomposite between these two elementary polymers. While polymer-silica composites are well known, the nanometric physical blending of PE and silica has remained a challenge. A method for the preparation of such materials, which is based on the entrapment of dissolved PE in a polymerizing tetraethoxysilane (TEOS) system, has been developed. Specifically, the preparation of submicron particles of low-density PE at silica and high-density PE at silica is detailed, which is based on carrying out a silica sol-gel polycondensation process within emulsion droplets of TEOS dissolved PE, at elevated temperatures. The key to the successful preparation of this new composite has been the identification of a surfactant, PE-b- PEG, that is capable of stabilizing the emulsion and promoting the dissolution of the PE. A mechanism for the formation of the particles as well as their inner structure are proposed, based on a large battery of analyses, including transmission electron microscopy (TEM) and scanning electron microscopies (SEM), surface area and porosity analyses, various thermal analyses including thermal gravimetric analysis (TGA/DTA) and differential scanning calorimetry (DSC) measurements, small-angle X-ray scattering (SAXS) measurements and solid-state NMR spectroscopy.
[27] H.Sertchook, Hi. Elimelech, C. Makarov, R. Khalfin, Journal of the American Chemical Society. 2007, 129, 98-108 |
Polyethylene (PE) and silica are perhaps the simplest and most common organic and inorganic polymers, respectively. We describe, for the first time, a physically interpenetrating nanocomposite between these two elementary polymers. While polymer-silica composites are well known, the nanometric physical blending of PE and silica has remained a challenge. A method for the preparation of such materials, which is based on the entrapment of dissolved PE in a polymerizing tetraethoxysilane (TEOS) system, has been developed. Specifically, the preparation of submicron particles of low-density PE@silica and high-density PE@silica is detailed, which is based on carrying out a silica sol-gel polycondensation process within emulsion droplets of TEOS dissolved PE, at elevated temperatures. The key to the successful preparation of this new composite has been the identification of a surfactant, PE-b-PEG, that is capable of stabilizing the emulsion and promoting the dissolution of the PE. A mechanism for the formation of the particles as well as their inner structure are proposed, based on a large battery of analyses, including transmission electron microscopy (TEM) and scanning electron microscopies (SEM), surface area and porosity analyses, various thermal analyses including thermal gravimetric analysis (TGA/DTA) and differential scanning calorimetry (DSC) measurements, small-angle X-ray scattering (SAXS) measurements and solid-state NMR spectroscopy. |
The source is mentioned, but it is not clear that the entire paragraph is taken from it verbatim. |
|
| [16.] Mrs/Fragment 012 25 - Diskussion Bearbeitet: 16. May 2015, 19:48 WiseWoman Erstellt: 29. March 2015, 09:46 (SleepyHollow02) | BauernOpfer, Fragment, Garcia et al 2004, Gesichtet, Mrs, SMWFragment, Schutzlevel sysop |
|
|
| Untersuchte Arbeit: Seite: 12, Zeilen: 25-32 |
Quelle: Garcia et al 2004 Seite(n): 169, Zeilen: abstract |
|---|---|
| M. Garcia et al. [55] have reported preparation of Polypropylene-SiO2 nanocomposites using twin-screw extruders. The properties of the nanocomposites were studied using two different inorganic fillers: colloidal (sol) and powdered silica nanoparticles. Reinforcing and toughening effects of the nanoparticles on the polymer matrix were found at a loading of 4.5 wt. %, which is lower than for most particulate filled composites. The use of silica nanoparticles led to different microstructure when compared with that of the pure polymer. Addition of colloidal silica to the polymer matrix produced good filler dispersion while the use of powdered silica resulted in aggregated silica particles in the polymer [matrix.]
[55] M.Garcia, G.van Vliet, S.Jain, Review on Advanced Materials Science 6 (2004) 169- 175 PP |
Polypropylene-SiO2 nanocomposites were synthesized using twin-screw extruders. The properties of the nanocomposites were studied using two different inorganic fillers: colloidal (sol) and powder silica nanoparticles. Reinforcing and toughening effects of the nanoparticles on the polymer matrix were found at a loading of 4.5 wt.%, which is lower than for most particulate filled composites. The use of silica nanoparticles led to different microstructure when compared with that of the pure polymer. Addition of colloidal silica to the polymer matrix produced good filler dispersion while the use of powder silica resulted in aggregated silica particles in the polymer matrix. |
The source is given, but it is not clear to the reader that simply the abstract of the paper described has been copied. The reference given is also incomplete. |
|
| [17.] Mrs/Fragment 013 01 - Diskussion Bearbeitet: 16. May 2015, 19:52 WiseWoman Erstellt: 29. March 2015, 09:50 (SleepyHollow02) | BauernOpfer, Fragment, Garcia et al 2004, Gesichtet, Mrs, SMWFragment, Schutzlevel sysop |
|
|
| Untersuchte Arbeit: Seite: 13, Zeilen: 1-4 |
Quelle: Garcia et al 2004 Seite(n): 169, Zeilen: abstract |
|---|---|
| There was no noticeable improvement of the mechanical properties when powder silica was added to the pure polymer. On the contrary, the presence of silica-sol nano-particles in the polymer matrix led to an increase of both Young modulus and impact strength, from 1.2 GPa to 1.6 GPa and from 3.4 kJ/m2 to 5.7 kJ/m2, respectively. | There was no noticeable improvement of the mechanical properties when powder silica was added to the pure polymer. On the contrary, the presence of silica-sol nano-particles in the polymer matrix led to an increase of both Young modulus and impact strength, from 1.2 GPa to 1.6 Gpa and from 3.4 kJ/m2 to 5.7 kJ/m2 respectively. |
The passage starts on the previous page: Mrs/Fragment 012 25 The source is given, but it is not clear to the reader that simply the abstract of the paper described has been copied. |
|
| [18.] Mrs/Fragment 013 06 - Diskussion Bearbeitet: 25. April 2015, 22:30 WiseWoman Erstellt: 4. January 2015, 13:13 (Klgn) | Fragment, Gesichtet, Mrs, SMWFragment, Schutzlevel sysop, Verschleierung, Yao and Yang 2010 |
|
|
| Untersuchte Arbeit: Seite: 13, Zeilen: 6-22 |
Quelle: Yao and Yang 2010 Seite(n): 492, Zeilen: r. Sp.; 6-11; Abstract |
|---|---|
| Poly (trimethylene terephthalate) (PTT) is a relatively new type of linear aromatic polyester. During recent decades, it has attracted attention not only because of its excellent mechanical and electrical properties, but also because one of its raw materials, 1,3-propanediol, is a renewable resource, which can be derived from renewable materials such as corn and starch. The sol–gel technique has provided promising opportunities for the preparation of polymer/inorganic hybrid materials at the molecular level, which ensures the inorganic particles, are well dispersed in the organic matrix. In this study [38], poly (trimethylene terephthalate) (PTT)/silica nanocomposites were fabricated via the sol–gel technique and in-situ polycondensation. Fourier transform infrared and nuclear magnetic resonance analyses confirmed that some PTT molecular chains were grafted to the surface of silica. Unlike pure PTT, the grafted PTT was insoluble in a mixed solvent of chloroform and hexafluoro-2-propanol. Both transmission electron microscopy and scanning electron microscopy showed that the silica particles, with a size of 40–50 nm, were homogeneously dispersed in the PTT matrix with no preferential accumulation in any region. Differential scanning calorimetry revealed that the glass transition temperature and cold-crystallization peak of the composites gradually increased with increasing silica loading. A simultaneous increase of stiffness and toughness was observed for the concentration of nanocomposites.
[38] ISO 2076, AUSG. 12.89: Generic names for man-made fibers. International Organization for Standardization |
Poly(trimethylene terephthalate) (PTT) is a relatively new type of linear aromatic polyester. During recent decades, it has attracted attention not only because of its excellent mechanical and electrical properties, 20 but also because one of its raw materials, 1,3-propanediol, is a renewable resource, which can be derived from renewable materials such as corn and starch.
[Abstract] The sol–gel technique has provided promising opportunities for the preparation of polymer/inorganic hybrid materials at the molecular level, which ensures the inorganic particles are well dispersed in the organic matrix. In this work, poly(trimethylene terephthalate) (PTT)/silica nanocomposites were fabricated via the sol–gel technique and in situ polymerization. Fourier transform infrared and nuclear magnetic resonance analyses confirmed that some PTT molecular chains were grafted to the surface of silica. Unlike pure PTT, the grafted PTT was insoluble in a mixed solvent of chloroform and hexafluoro-2-propanol. Both transmission electron microscopy and scanning electron microscopy showed that the silica particles, with a size of 40–50 nm, were homogeneously dispersed in the PTT matrix with no preferential accumulation in any region. Differential scanning calorimetry revealed that the glass transition temperature and cold-crystallization peak of the composites gradually increased with increasing silica loading. A simultaneous increase of stiffness and toughness was observed for the nanocomposites. [20] Wu J, Schultz JM, Samon JM, Pangelinan AB and Chuah HH, Polymer 42:7141 (2001). |
|
|
| [19.] Mrs/Fragment 013 25 - Diskussion Bearbeitet: 25. April 2015, 22:41 WiseWoman Erstellt: 24. January 2015, 17:27 (Klgn) | Bhattacharya et al. 2008, Fragment, Gesichtet, Mrs, SMWFragment, Schutzlevel sysop, Verschleierung |
|
|
| Untersuchte Arbeit: Seite: 13, Zeilen: 25-31 |
Quelle: Bhattacharya et al. 2008 Seite(n): 28; 29, Zeilen: 28: 27 ff.; 29: 3-4; 28: 8 ff. |
|---|---|
| Polyurethane (PU) is becoming increasingly important as an engineering material because it has excellent abrasion resistance and displays properties of both elastomers and plastics. Wang and Pinnavaia [29] have synthesized intercalated nanocomposites based on elastomeric polyurethane. Khudyakov and Zopf [49] also studied the effect of colloidal silica and organoclays on properties of PU. The studies on epoxy systems considered the ring opening polymerization of epoxides to form polyether nanocomposites. Studies [30, 31] of both rubbery and glassy epoxy/clay nanocomposites using different type of amine curing agents were [conducted and the mechanisms leading to the mono-layer exfoliation of clay layers in thermoset epoxy systems were elucidated.]
[30] P.B., Messersmith, E.P. Giannelies, Chemical Matterial 6, (1994), 1719-1725 [31] T. Lan, T.J. Pinnavaia, Chemical Materials, 6, 1994, 2216- 2219 |
[page 28]
Polyurethane (PU) is becoming increasingly important as an engineering material because it has excellent abrasion resistance and displays properties of both elastomers and plastics. [page 29] [Wang and Pinnavaia (1998)] have synthesized intercalated nanocomposites based on elastomeric polyurethanes. [page 28] The studies on epoxy systems considered the ring opening polymerization of epoxides to form polyether nanocomposites. Studies of both rubbery and glassy epoxy/clay nanocomposites using different types of amine curing agents were conducted and the mechanisms leading to the monolayer exfoliation of clay layers in thermoset epoxy systems were elucidated. |
|
|
| [20.] Mrs/Fragment 014 01 - Diskussion Bearbeitet: 16. May 2015, 17:50 Hindemith Erstellt: 24. January 2015, 17:52 (Klgn) | Bhattacharya et al. 2008, Fragment, Gesichtet, Mrs, SMWFragment, Schutzlevel sysop, Verschleierung |
|
|
| Untersuchte Arbeit: Seite: 14, Zeilen: 1ff (entire page) |
Quelle: Bhattacharya et al. 2008 Seite(n): 27-29, Zeilen: 27: 19 ff.28: 9-12; 29: 10 ff. |
|---|---|
| [Studies [30, 31] of both rubbery and glassy epoxy/clay nanocomposites using different type of amine curing agents were] conducted and the mechanisms leading to the mono-layer exfoliation of clay layers in thermoset epoxy systems were elucidated.
2.2.3. Elastomers Burnside and Giannelis [32] have described the two-step preparation of silicon rubber-based nanocomposites. First, silanol-terminated poly (dimethyl siloxane) (PDMS) was blended at room temperature with dimethyl-ditallow ammonium-exchanged montmorillonite, followed by crosslinking of the silanol end groups with tetra ethyl- orthosilicate (TEOS) in the presence of bis(2-ethylhexanoate) as catalyst at room temperature. Also Okada and co-worker [33] obtained a nitrile rubber (NBR) – based nanocomposite in a dual-step synthesis. 2.2.4. Natural and biodegradable polymers Natural and biodegradable polymers are a new generation of polymers that are relatively friendly to the environment with little or no impact when disposed. Such polymers include polylactide (PLA), starch, and cellulose among others. Although these polymers are considered to be environmentally-friendly, they have relatively weak mechanical properties, such as brittleness, low heat distortion, low tensile strength. And their use in packaging is limited due to high gas permeability [34]. Addition of nano-scale fillers has been shown to improve these properties significantly, allowing these polymers to be used in applications such as disposable food service items, food packaging, health care product, packing foams and agricultural mulch film. PLA polymers are linear aliphatic polyester, generally produced by ring-opening polymerization of lactide dimer. Sinha Ray and Okamoto [35] have shown that the addition of nanoscale modified montmorillonite increased both solid and melt state properties, such as flexural properties, rheological properties, reduced gas permeability and increased rate of biodegradability. Ogata et al. [36] also reported similar enhancement of properties. Another biodegradable polymer is polycaprolactone (PCL), linear polyester manufactured by ring-opening polymerization of ε-caprolactone. The PCL chain is flexible and exhibits high elongation at break and low modulus. Its physical properties make it very attractive, not only as a substitute material for nondegradable polymer but also as a plastic material for medical and agricultural applications. The main drawback of PCL is its low melting point (65°C ), which can be overcome by blending it with other polymers. Many attempts to prepare PCL nanocomposites with much improved mechanical and materials properties than that of neat PCL have been reported [34]. [30] P.B., Messersmith, E.P. Giannelies, Chemical Matterial 6, (1994), 1719-1725 [31] T. Lan, T.J. Pinnavaia, Chemical Materials, 6, 1994, 2216- 2219 [32] S.D., Burnside, E.P. Giannelis, Chemical Materials, 7, (1995), 1597-1600 [33] A. Okada, K. Fujumori, A. Usuki, Polymer Chemistry, 32,(2), 540-541, 1991 [34] S. Sinha Ray, M. Bousmina, Progress in Material Science, 50, 962-1079, 2005 [35] S. Sinha Ray, M. Okamoto, Macromolecule Rapid Communication,24 (14), 815- 840, 2003 [36] N. Ogata, G. jimentz, Journal of polymer science Part B, 35 (2), 389- 396, 1997 |
[S. 28]
Studies of both rubbery and glassy epoxy/clay nanocomposites using different types of amine curing agents were conducted and the mechanisms leading to the monolayer exfoliation of clay layers in thermoset epoxy systems were elucidated. [S.27] 2.3.2 Elastomers [Burnside and Giannelis (1995)] have described the two-step preparation of silicon rubber- based nanocomposites. First, silanol-terminated poly(dimethyl siloxane) (PDMS, Mw= 18000) was melt blended at room temperature with dimethyl-ditallow ammonium- exchanged montmorillonite, followed by cross-linking of the silanol end groups with tetraethyl-orthosilicate (TEOS) in the presence of bis(2-ethylhexanoate) as catalyst at room temperature. [...] [Okada and co-workers (1990), (1991)] obtained a nitrile rubber (NBR)-based nanocomposite in a dual-step synthesis. [S. 29]
Natural and biodegradable polymers are a new generation of polymers that are relatively friendly to the environment with little or no impact when disposed. Such polymers include polylactide (PLA), starch, and cellulose among others. Although these polymers are considered to be environmentally-friendly, they have relatively weak mechanical properties, such as brittleness, low heat distortion, low tensile strength, and their use in packaging is limited due to high gas permeability [Sinha Ray and Bousmina (2005)]. Addition of nanoscale fillers has been shown to improve these properties significantly, allowing these polymers to be used in applications such as disposable food service items, food packaging, health care products, packing foams and agricultural mulch film. PLA polymers are linear aliphatic polyesters, generally produced by ring-opening polymerization of lactide monomers. According to [Sinha Ray and Okamoto (2003)], their mechanical properties, thermal plasticity and biocompatibility are generally good and have much promise in many applications. They have, however, shown that the addition of nanoscale modified montmorillonite increased both solid and melt state properties, such as flexural properties, rheological properties, reduced gas permeability and increased rate of biodegradability. [...] Polycaprolactone (PCL) is a linear polyester manufactured by ring-opening polymerization of ε-caprolactone. [...] The PCL chain is flexible and exhibits high elongation at break and low modulus. Its physical properties and commercial availability make it very attractive, not only as a substitute material for nondegradable polymers for commodity applications, but also as a plastic material for medical and agricultural applications. The main drawback of PCL is its low melting point (65°C), which can be overcome by blending it with other polymers or by radiation cross-linking processes resulting in enhanced properties for a wide range of applications. Many attempts to prepare PCL nanocomposites with much improved mechanical and materials properties than that of neat PCL have been reported [Ray and Bousmina (2005)]. |
The source is not mentioned. All references to the literature except one have been taken from the source. |
|
| [21.] Mrs/Fragment 015 04 - Diskussion Bearbeitet: 2. May 2015, 20:36 WiseWoman Erstellt: 24. January 2015, 18:20 (Klgn) | Bhattacharya et al. 2008, Fragment, Gesichtet, Mrs, SMWFragment, Schutzlevel sysop, Verschleierung |
|
|
| Untersuchte Arbeit: Seite: 15, Zeilen: 4-22 |
Quelle: Bhattacharya et al. 2008 Seite(n): 25, Zeilen: 6-26 |
|---|---|
| The two major types of polyamides are polyamide 6 and polyamide 66. PA 6 is prepared by the polymerization of caprolactam. PA 66 is derived from the polycondensation of hexamethyelene diamine with adipic acid. Polyamides are crystalline polymers. The key features include a high degree of solvent resistance, thoughness, and fatigue resistance. Polyamides do exhibit a tendency to creep under applied load. Glass fibers and mineral fillers are often used to enhance the properties of polyamides. In addition, the properties of polyamides are greatly affected by moisture. The largest area of application for polyamide is in fiber and engineering plastic. Molded applications include automotive component, related machine parts (gear, cams, pulleys, rollers, etc.), and electrical insulation.
Earlier studies [79] have illustrated that the addition of clay to PA has improved the strength, stiffness, barrier, and heat resistance properties of polyamide 6. The barrier resins exhibit reduced moisture absorption and increased melt stability. Toyota researchers (1989) have shown that, similar to other nanocomposites, PA nanocomposites are able to achieve a lot of improved characteristics compared to pure PA. Thus, it has been reported that PA6 nanocomposites show higher tensile strength, higher tensile modulus, higher heat distortion temperature, increased solvent resistance, decreased thermal expansion coefficient, reduced gas permeability and increased flame retardancy. With these enhanced properties, PA nanocomposites have found increased application in the automobile and textile industries, where stronger yarn could be produced, with better extensional characteristics [88-90]. |
The two major types of polyamides are nylon 6 and nylon 66. Nylon 6, or polycaprolactam, is prepared by the polymerization of caprolactam. Poly (hexamethylene adipamide), or nylon 66, is derived from the condensation polymerization of hexamethylene diamine with adipic acid. Polyamides are crystalline polymers. Their key features include a high degree of solvent resistance, toughness, and fatigue resistance. Nylons do exhibit a tendency to creep under applied load. Glass fibers or mineral fillers are often used to enhance the properties of polyamides. In addition, the properties of nylon are gready affected by moisture. The largest area of application for nylons is in fibers. Molded applications include automotive components, related machine parts (gears, cams, pulleys, rollers, boat propellers, etc.), appliance parts, and electrical insulation.
Earlier studies have illustrated that the addition of clay to PA has improved the strength, stiffness, barrier, and heat resistance properties of nylon 6. The barrier resins exhibit reduced moisture absorption and increased melt stability. Toyota researchers (1989) have shown that, similar to other nanocomposites, PA nanocomposites are able to achieve much improved characteristics compared to neat PA. It has been reported that PA6 nanocomposites show approximately 40 % higher tensile strength, 68 % higher tensile modulus, 60 % higher flexural strength, 126 % higher flexural modulus, higher heat distortion temperatures, increased solvent resistance, decreased thermal expansion coefficient, reduced gas permeability, and increased flame retardancy. With these enhanced properties, PA nanocomposites have found increased application in the automobile and textile industries, where stronger yarns could be produced, with better extensional characteristics. |
|
|
| [22.] Mrs/Fragment 024 11 - Diskussion Bearbeitet: 16. May 2015, 14:21 Hindemith Erstellt: 29. March 2015, 09:59 (SleepyHollow02) | Fragment, Gesichtet, Monserrat De La Luz Garcia Curiel 2004, Mrs, SMWFragment, Schutzlevel sysop, Verschleierung |
|
|
| Untersuchte Arbeit: Seite: 24, Zeilen: 11-14, 18-23 |
Quelle: Monserrat De La Luz Garcia Curiel 2004 Seite(n): 13, 14, Zeilen: 13: 39 ff.; 14: 1, 9 ff. |
|---|---|
| 2.4. Nanoparticle based nanocomposite
Inorganic particles are used in different matrices for specific purposes [54]. For metals, fillers improve high temperature creep properties and hardness when compared with the pure metal. For ceramics, fillers are used to improve their toughness [53] and for polymers for the increase of stiffness, strength, electrical properties and occasionally for toughness. [...] The incorporation of fillers into organic polymers may result in a brittle composite material. In addition, the amount of filler that can be incorporated is limited (thus, sometimes the addition of higher amounts of filler does not improve the mechanical properties of the material) and the filler may not be uniformly dispersed in the organic polymer. The efficiency of the filler to modify the properties of the polymer is primarily determined by the degree of dispersion in the polymer matrix. [53] Callister, W.D. Materials Science and Engineering. An Introduction. 5th Ed. John Wiley & Sons, Inc. (1999). [54] Wypych, G. Handbook of fillers. 2nd Edition. New York (1999). |
Inorganic particles are used in different matrices for specific purposes [43]. For metals, fillers improve high temperature creep properties and hardness when compared with the pure metal. For ceramics, fillers are used to improve their toughness [35] and for polymers for the increase of stiffness, strength, electrical properties and occasionally for
[page 14] toughness. [...] Unfortunately, the incorporation of fillers in organic polymers can result in a brittle composite material. In addition, the amount of filler that can be incorporated is limited (sometimes the addition of higher amounts of filler doest not improve the mechanical properties of the material) and the filler may not be uniformly dispersed in the organic polymer. The efficiency of the filler to modify the properties of the polymer is primarily determined by the degree of dispersion in the polymer matrix. [35] Callister, W.D. Materials Science and Engineering. An Introduction. 5th Ed. John Wiley & Sons, Inc. (1999). [43] Wypych, G. Handbook of fillers. 2nd Edition. New York (1999). |
The source is not mentioned, the references to the literature are also copied. |
|
| [23.] Mrs/Fragment 025 20 - Diskussion Bearbeitet: 16. May 2015, 14:27 Hindemith Erstellt: 29. March 2015, 10:05 (SleepyHollow02) | Fragment, Gesichtet, Monserrat De La Luz Garcia Curiel 2004, Mrs, SMWFragment, Schutzlevel sysop, Verschleierung |
|
|
| Untersuchte Arbeit: Seite: 25, Zeilen: 20-22, 25-28 |
Quelle: Monserrat De La Luz Garcia Curiel 2004 Seite(n): 15, 16, Zeilen: 15:36 ff. ; 16: 1 ff. |
|---|---|
| 2.4.2. Silica
Silica has been used in different polymers as a reinforcement material. Examples are in methacrylate [61-72], polyimide [6, 73], polyamide [74], rubbery epoxies [75], and acrylic [76]. [...] The specific function of the filler is based on the specific resin system, particle size, surface area, loading and surface modification. Because of the high bond energy in the Si-O bond, SiO2 has extremely high thermal stability. SiO2 also possesses a very low thermal expansion coefficient. [6] C. Joly, M. Smaihi, L. Porcar, Chemistry of Materials, 11, 2331 (1999). [61] M.M. Hasan, Y. Zhou, H. Mahfuz, Materials Science and Engineering A 429 (2006) 181– 188 [62] F.Hussain, M.Hojjati, M.Okamoto, Journal of COMPOSITE MATERIALS, Vol. 40, No. 17/2006, and references therein. [63] Zheng, Y.P., Zheng Y. and Ning, R.C. (2003, Materials Letters, 57(19): 2940–2944. [64] Tang, J., Wang, Y., Liu, H., Xia, Y. and Schneider, B. (2003), J. of Applied Polymer Science, 90: 1053–1057. [65] M.W.L. Wilbrink, A.S. Argon, R.E. Cohen, M. Weinberg, Polymer 42 (26) (2001) 10155– 10180. [66] H. Mahfuz, V.K. Rangari, M.S. Islam, S. Jeelani, Compos. Part A: Appl. Sci. Manuf. 35 (4) (2004) 453–460. [67] G. Chen, G. Luo, X. Yang, Y. Sun, J. Wang, Mater. Sci. Eng. A 380 (1–2) (2004) 320– 325. [68] N. Chisholm, H. Mahfuz, V.K. Rangari, A. Ashfaq, S. Jeelani, Compos. Struct. 67 (1) (2005) 115–124. [69] M.Z. Rong, M.Q. Zhang, Y.X. Zheng, H.M. Zeng, K. Friedrich, Polymer 42 (7) (2001) 3001–3004, 3001.98 [70] X. Li, G. Wang, X. Li, Surf. Coatings Technol. 197 (1) (2005) 56–60. [71] G. Vigier, J. Pascualt, J. Gerard, L. David, and Haiji, Journal of Polymer Science. 37, 3172 (1999) [72] Ch. Landry, and B. Coltrain, Polymer 33, 7 (1992). [73] Y. Yang, J. Yin, Z. Qi, and Z. Zhu, Journal of Applied Polymer Science 73, 2977 (1999). [74] F. Yang, Y.Ou, and Z. Yu, Journal of Applied Polymer Science 69, 355 (1998). [75] J. Kolarik, O. Dukh, L. Matejka, Polymer 41, 1449 (2000). [76] K. Qiu, and Z. Huang, Polymer 38, 521 (1997). [77] E. Werner, van Zyl, G. Monserrat, Macromolecular Materials and Engineering, 2002, 287, 106-110, and references therein. [78] E. P. Giannelis, Advanced Materials 1996, 8, 29. |
Silica
Silica has been used in different polymers as a reinforcement material. Examples are in methacrylate [70-73], polyimide [74-75], polyamide [76], rubbery epoxies [77], and acrylic [78]. The specific function of the filler is based on the specific resin system, particle size, surface area, loading and surface modification. Because of the high bond energy in the Si-O bond, SiO2 has extremely high [page 16] thermal stability. SiO2 also possesses a very low thermal expansion coefficient. [70] Brinker, J. and Scherer, G. Sol-Gel Science, Academic Press (1990). D.H. Everett, Basic principles of colloid science Ed. The Royal Society of Chemistry, print. Whitstable, Kent, UK (1988). [71] Vigier, G., Pascualt, J., Gerard, J., David, L. and Haiji, J. Polym. Sci. 37, 3172 (1999). [72] Mallouk, T., Ollivier, J. and Johnson, S. Science 283 (1999). [73] Landry, Ch. and Coltrain, B. Polymer 33, 7 (1992). [74] Smaihi, M., Joly, C. and Noble, R. Chem. Mater. 11, 2331 (1999). [75] Yang, Y., Yin, J., Qi, Z. and Zhu, Z. J. Appl. Polym. Sci. 73, 2977 (1999). [76] Yang, F., Ou, Y. and Yu, Z.-Z. J. Appl. Polym. Sci. 69, 355 (1998). [77] Kolarik, J., Dukh, O., Matejka, L. Polymer 41, 1449 (2000). [78] Qiu, k. and Huang, Z. Polymer 38, 521 (1997). |
The source is not mentioned. |
|
| [24.] Mrs/Fragment 026 02 - Diskussion Bearbeitet: 25. April 2015, 22:24 WiseWoman Erstellt: 28. December 2014, 21:55 (Hindemith) | Fragment, Gesichtet, Mrs, SMWFragment, Schutzlevel sysop, Van Zyl et al 2002, Verschleierung |
|
|
| Untersuchte Arbeit: Seite: 26, Zeilen: 2-13 |
Quelle: Van Zyl et al 2002 Seite(n): 106, 107, Zeilen: 106: l.col: 2 ff.; 107: l.col: 1 ff. |
|---|---|
| Polymer nanocomposites constitute a class of hybrid materials composed of a polymer matrix and an inorganic filler component which has at least one dimension in the nanometer (100 nm) size domain [77- 79]. The properties of a polymer-reinforced composite are mostly influenced by the size, shape, composition, state of agglomeration, and degree of matrix filler adhesion [80]. Optimum surface curvature at the polymer-filler interface can be realized when large surface areas are created, which is possible when the filler particles are sufficiently small [81]. Decreasing the particle size to the nano-size dimension influences the macroscopic properties of the polymer because a breakdown of the common rule-of-mixture theory occurs [82]. A major challenge remains, however, to effectively incorporate mono-disperse nanoparticles inside a polyamide matrix since a drawback of such small particles is their tendency to aggregate, particularly when higher particle concentrations, intimate mixing and prolonged heating are part of the reaction conditions.
[77] E. Werner, van Zyl, G. Monserrat, Macromolecular Materials and Engineering, 2002, 287, 106-110, and references therein. [78] E. P. Giannelis, Advanced Materials 1996, 8, 29. [79] P. C. le Baron, Z. Wang, T. J. Pinnavaia, Applied Clay Science 1999, 15, 11 [80] W. Helbert, J. Y. Cavaille, A. Dufresne, Polymer Composites 1996, 17, 604. [81] D. W. Clegg, A. A. Collyer, ªMechanical Properties of Reinforced Thermoplasticsº, Elsevier 1986. [82] J. Choi, J. Harcup, A. F. Yee, Q. Zhu, R. M. Laine, Journal of the American Chemical Society. 2001, 123, 11420, |
Polymer nanocomposites constitute a class of hybrid materials composed of a polymer matrix and an inorganic filler component which has at least one dimension in the nanometer (<100 nm) size domain.[1, 2] [...] The properties of a polymer-reinforced composite are mostly influenced by the size, shape, composition, state of agglomeration, and degree of matrix-filler adhesion.[4] Optimum surface curvature at the polymer-filler interface can be realized when large surface areas are created, which is possible when the filler particles are sufficiently small.[5] Decreasing the particle size to the nano-size dimension influences the macroscopic properties of the polymer because a breakdown of the common rule-of-mixture theory occurs.[6] [...] A major challenge remains, however, to effectively incorporate monodisperse nanoparticles inside a polyamide
[page 107] matrix since a drawback of such small particles is their tendency to aggregate, particularly when higher particle concentrations, intimate mixing and prolonged heating are part of the reaction conditions. [1] E. P. Giannelis, Adv. Mater. 1996, 8, 29. [2] [2a] ªPolymer Clay Nanocompositesº, T. J. Pinnavaia, G. W. Beall, Eds., Wiley, New York 2001; [2b] P. C. le Baron, Z. Wang, T. J. Pinnavaia, Appl. Clay Sci. 1999, 15, 11. [4] W. Helbert, J. Y. Cavaille, A. Dufresne, Polym. Comp. 1996, 17, 604. [5] D. W. Clegg, A. A. Collyer, ªMechanical Properties of Reinforced Thermoplasticsº, Elsevier 1986. [6] J. Choi, J. Harcup, A. F. Yee, Q. Zhu, R. M. Laine, J. Am. Chem. Soc. 2001, 123, 11420, and references therein. |
The source is mentioned as one of three references for the first sentence. It does not become clear that the entire passage, including all references, is taken from the source. |
|
| [25.] Mrs/Fragment 026 17 - Diskussion Bearbeitet: 2. May 2015, 20:38 WiseWoman Erstellt: 24. January 2015, 18:33 (Klgn) | BauernOpfer, Bhattacharya et al. 2008, Fragment, Gesichtet, Mrs, SMWFragment, Schutzlevel sysop |
|
|
| Untersuchte Arbeit: Seite: 26, Zeilen: 17-22 |
Quelle: Bhattacharya et al. 2008 Seite(n): 16, Zeilen: 36-41 |
|---|---|
| 2.5.1. Melt intercalation
Melt intercalation is the most widely used method in polymer/ particle nanocomposite preparation, and it has tremendous potential for industrial application. An advantage of this method over the others is that no solvent is required. The melt intercalation process involves mixing the particles by annealing, statically or under shear, with polymer pellets while heating the mixture above the melting point of the polymer [8]. [8] S. N.Bhattacharya, Rahul K. Gupta, Polymeric Nanocomposites Theory and Practice, Hanser 2008 |
2.2.3 Melt Intercalation
Melt intercalation is the most widely used method in polymer/clay nanocomposite preparation, and it has tremendous potential for industrial application. An advantage of this method over the others is that no solvent is required. The melt blending process involves mixing the layered silicate by annealing, statically or under shear, with polymer pellets while heating the mixture above the softening point of the polymer. |
|
|
| [26.] Mrs/Fragment 027 08 - Diskussion Bearbeitet: 2. May 2015, 20:39 WiseWoman Erstellt: 24. January 2015, 18:53 (Klgn) | BauernOpfer, Bhattacharya et al. 2008, Fragment, Gesichtet, Mrs, SMWFragment, Schutzlevel sysop |
|
|
| Untersuchte Arbeit: Seite: 27, Zeilen: 8-13 |
Quelle: Bhattacharya et al. 2008 Seite(n): 15, Zeilen: 7-13 |
|---|---|
| 2.5.3. in-situ polymerization
In -situ polymerization involves the dispersion and distribution of particle in the monomer followed by polymerization. The particle is swollen within the liquid monomer or a monomer solution so that polymer nanocomposite formation can occur during polymerization process. Polymerization can be initiated either by heat or radiation, diffusion of a suitable initiator, or by an organic initiator [8]. [8] S. N.Bhattacharya, Rahul K. Gupta, Polymeric Nanocomposites Theory and Practice, Hanser 2008 |
2.2.2 In-Situ Polymerization
In-situ polymerization involves the dispersion and distribution of clay layers in the monomer followed by polymerization. The layered silicate is swollen within the liquid monomer or a monomer solution so that polymer formation can occur between the intercalated sheets. Polymerization can be initiated either by heat or radiation, diffusion of a suitable initiator, or by an organic initiator or catalyst fixed through cation exchange inside the interlayer before the swelling step. |
|
|
| [27.] Mrs/Fragment 029 11 - Diskussion Bearbeitet: 19. April 2015, 18:53 WiseWoman Erstellt: 28. December 2014, 00:57 (Hindemith) | BauernOpfer, Fragment, Gesichtet, Mahfuz et al 2008, Mrs, SMWFragment, Schutzlevel sysop |
|
|
| Untersuchte Arbeit: Seite: 29, Zeilen: 11-23 |
Quelle: Mahfuz et al 2008 Seite(n): 27, Zeilen: 27 ff. |
|---|---|
| Polyamide’s toughness, low coefficient of friction and good abrasion resistance make it an ideal replacement for a wide variety of applications, replacing metal and rubber. The amide groups of polyamide are very polar, and hydrogen bonds can form between them. Because of these, and because the polyamide backbone is so regular and symmetrical, polyamides are often crystalline, and make very good fibers [107]. When polyamide is spun into fibers, the long chainlike macromolecules line up parallel to each other. The amide groups on adjacent chains then form strong bonds with each other called bridged hydrogen bonds. These hydrogen bonds hold the adjacent chains together, making polyamide yarn strong. When polyamide 6 polymerizes, the amide link present in caprolactam (starting monomer for polyamide 6) opens up and the molecules join up in a continuous chain, providing an ideal mechanism for interacting with nanoparticles. On the other hand, silica particles are formed by strong covalent bonds between silicon and oxygen atoms by sharing their electron pairs at the p orbital. The surface bound OH groups on the silica surfaces may also form stable bonds with polyamide during polymerization.
[107] H. Mahfuz, M. Hasan, V. Dhanak, Nanotechnology 19 (2008) 445702 (7pp) |
Nylon’s toughness, low coefficient of friction and good abrasion resistance make it an ideal replacement for a wide variety of applications replacing metal and rubber. The amide groups of nylon are very polar, and can hydrogen bond with each other. Because of these, and because the nylon backbone is so regular and symmetrical, nylons are partially crystalline, and they make very good fiber [22-26].[...] When nylon is spun into fibers, the long chain-like macromolecules line up parallel to each other. The amide groups on adjacent chains then form strong bonds with each other called hydrogen bonds. These hydrogen bonds hold the adjacent chains together, making nylon yarn strong. When nylon-6 polymerizes, the amide link present in Caprolactam (starting monomer for nylon-6) opens up and the molecules join up in a continuous chain providing an ideal mechanism for interacting with nanoparticles. On the other hand, silica particles are formed by strong covalent bonds between silicon and oxygen atoms by sharing their electron pairs at the p orbitals. In addition, the surface bound OH groups on silica surfaces offer an opportunity to form stable bonds with nylon or any functional group during polymerization.
[22] Kohan M. I, editor. Nylon Plastics, John Wiley & Sons, New York 1973, p.3-4. [23] Fornes TD, Paul D.R., Polymer, 44, 3945, ., 2003 [24] Kim, Young, Materials Research Society Symposium - Proceedings, 2002, V740, 441 [25] Zhang, Wei-De, Macromolecular Rapid Communications, 2004,V 25, n 21, 1860. [26] Jin L, Bower C, Zhou O. Appl Phys, 1998 Lett, 73, 1197. |
The source is mentioned (the source used for documentation here and the source given both contain the section used here). It does not become clear, however, that the entire passage has been taken from the source. |
|
| [28.] Mrs/Fragment 030 08 - Diskussion Bearbeitet: 16. May 2015, 13:29 Hindemith Erstellt: 29. March 2015, 09:08 (SleepyHollow02) | Fragment, Gesichtet, Holister et al 2003, Mrs, SMWFragment, Schutzlevel sysop, Verschleierung |
|
|
| Untersuchte Arbeit: Seite: 30, Zeilen: 8-23 |
Quelle: Holister et al 2003 Seite(n): 5, 6, Zeilen: 5: 23 ff.; 6: 1 ff., 31 ff. |
|---|---|
| The transition from microparticles to nanoparticles can lead to a number of changes in physical properties. Two of the major factors are the increase in the ratio of surface area to volume, and the size of the particle moving into the area where quantum effects predominate. The increase in the surface area to volume ratio, which is a gradual progression as the particle gets smaller, leads to an increasing dominance of the behavior of atoms located on the surface of a particle over that of those located in the interior of the particle. This affects both the properties of the particle as such and its interaction with other materials. High surface area is a critical factor in the performance of catalysis and structure such as electrodes, allowing improvement in performance of such technologies as fuel cells and batteries. The large surface area of nanoparticles also results in a lot of interaction between the intermixed materials in nanocomposites, leading to special properties such as increased strength and/or increased chemical and heat resistance.
Nanoparticles are currently made out of a very wide variety of materials, the most common of the new generation of nanoparticles being ceramics, which are best split into metal oxide ceramics such as titanium, zinc, aluminum and iron oxide, to name a prominent few, and silicate nanoparticle [108, 109]. [108] F. Luis, E. Mauricio, L.L. Betty, Macromol. Symp. 2007, 258, 119–128 [109] B. Salima, B. Elodie, Z. Nathalie, Macromol. Rapid Commun. 2005, 26, 1860–1865, |
The transition from microparticles to nanoparticles can lead to a number of changes in physical properties. Two of the major factors in this are the increase in the ratio of surface area to volume, and the size of the particle moving into the realm where quantum effects predominate.
The increase in the surface-area-to-volume ratio, which is a gradual progression as the particle gets smaller, leads to an increasing dominance of the behavior of atoms on the surface of a particle over that of those in the interior of the particle. This affects both the properties of the particle in isolation and its interaction with other materials. High surface area is a critical factor in the performance of catalysis and structures such as electrodes, allowing improvement in performance of such technologies as fuel cells and batteries. The [page 6] large surface area of nanoparticles also results in a lot of interactions between the intermixed materials in nanocomposites, leading to special properties such as increased strength and/or increased chemical/heat resistance. [...] Nanoparticles are currently made out of a very wide variety of materials, the most common of the new generation of nanoparticles being ceramics, which are best split into metal oxide ceramics, such as titanium, zinc, aluminum and iron oxides, to name a prominent few, and silicate nanoparticles (silicates, or silicon oxides, are also ceramic), generally in the form of nanoscale flakes of clay. |
The source is not given. |
|
| [29.] Mrs/Fragment 031 23 - Diskussion Bearbeitet: 16. May 2015, 10:50 Hindemith Erstellt: 29. December 2014, 08:31 (SleepyHollow02) | Fragment, Gesichtet, KomplettPlagiat, Mrs, SMWFragment, Schutzlevel sysop, Seiten, die magische ISBN-Links verwenden, Wikipedia Silicon dioxide 2011 |
|
|
| Untersuchte Arbeit: Seite: 31, Zeilen: 23-26, 27-29 |
Quelle: Wikipedia Silicon dioxide 2011 Seite(n): online, Zeilen: - |
|---|---|
| The chemical compound silicon dioxide, also known as silica (from the Latin silex), is an oxide of silicon with the chemical formula SiO2. It has been known for its hardness since antiquity. Silica is most commonly found in nature as sand or quartz, as well as in the cell walls of diatoms [113, 117]. [...] Silica is manufactured in several forms including fused quartz, crystal, fumed silica (or pyrogenic silica, trademarked Aerosil or Cab-O-Sil), colloidal silica, silica gel, and aerogel.
[113] R.K. Iler The chemistry of silica. New York: Wiley, 1979. [117] Lynn Townsend White, Jr. (1961). "Eilmer of Malmesbury, an Eleventh Century Aviator: A Case Study of Technological Innovation, Its Context and Tradition". Technology and Culture (Society for the History of Technology) 2 (2): 97–111. doi:10.2307/3101411. |
The chemical compound silicon dioxide, also known as silica (from the Latin silex), is an oxide of silicon with the chemical formula SiO2. It has been known for its hardness since antiquity. Silica is most commonly found in nature as sand or quartz, as well as in the cell walls of diatoms.[1][2] Silica is manufactured in several forms including fused quartz, crystal, fumed silica (or pyrogenic silica, trademarked Aerosil or Cab-O-Sil), colloidal silica, silica gel, and aerogel.
1. Iler, R.K. (1979). The Chemistry of Silica. Plenum Press. ISBN 047102404X. 2. Lynn Townsend White, Jr. (1961). "Eilmer of Malmesbury, an Eleventh Century Aviator: A Case Study of Technological Innovation, Its Context and Tradition". |
The source is not mentioned anywhere. |
|
| [30.] Mrs/Fragment 033 09 - Diskussion Bearbeitet: 16. May 2015, 11:00 Hindemith Erstellt: 28. March 2015, 13:15 (SleepyHollow02) | Fragment, Gesichtet, Mrs, SMWFragment, Schutzlevel sysop, Verschleierung, Wikipedia Silicon dioxide 2011 |
|
|
| Untersuchte Arbeit: Seite: 33, Zeilen: 9-18 |
Quelle: Wikipedia Silicon dioxide 2011 Seite(n): online, Zeilen: - |
|---|---|
| In the vast majority of silicates, the Si atom shows tetrahedral coordination, with 4 oxygen atoms surrounding a central Si atom. The most common example is seen in the quartz crystalline form of silica SiO2. In each of the thermodynamically most stable crystalline forms of silica, on average, all 4 of the vertices (or oxygen atoms) of the SiO4 tetrahedron are shared with others, yielding the net chemical formula: SiO2
Figure 3.2 Tetrahedral structural unit of silica (SiO4), the basic building block of the most ideal glass former The amorphous structure of glassy silica (SiO2) is in two-dimensions. No long-range order is present; however there is local ordering with respect to tetrahedral arrangement of oxygen (O) atoms around the silicon (Si) atoms. Note that a fourth oxygen atom is bonded to each silicon atom, either behind the plane of the screen or in front of it; these atoms are omitted for clarity. |

Tetrahedral structural unit of silica (SiO4), the basic building block of the most ideal glass former. In the vast majority of silicates, the Si atom shows tetrahedral coordination, with 4 oxygen atoms surrounding a central Si atom. The most common example is seen in the quartz crystalline form of silica SiO2. In each of the most thermodynamically stable crystalline forms of silica, on average, all 4 of the vertices (or oxygen atoms) of the SiO4 tetrahedra are shared with others, yielding the net chemical formula: SiO2. [...] The amorphous structure of glassy silica (SiO2) in two-dimensions. No long-range order is present; however there is local ordering with respect to the tetrahedral arrangement of oxygen (O) atoms around the silicon (Si) atoms. Note that a fourth oxygen atom is bonded to each silicon atom, either behind the plane of the screen or in front of it; these atoms are omitted for clarity. |
The source is not mentioned. |
|
| [31.] Mrs/Fragment 034 01 - Diskussion Bearbeitet: 16. May 2015, 11:08 Hindemith Erstellt: 28. March 2015, 13:24 (SleepyHollow02) | Fragment, Gesichtet, Mrs, SMWFragment, Schutzlevel sysop, Seiten, die magische ISBN-Links verwenden, Verschleierung, Wikipedia Silicon dioxide 2011 |
|
|
| Untersuchte Arbeit: Seite: 34, Zeilen: 1 ff. (entire page) |
Quelle: Wikipedia Silicon dioxide 2011 Seite(n): 1 (online source), Zeilen: - |
|---|---|

Figure 3.3 The amorphous structure of glassy silica (SiO2) in two-dimensions. Note that a fourth oxygen atom is bonded to each silicon atom, either behind the plane of the screen or in front of it; these atoms are omitted for clarity For example, in the unit cell of alpha-quartz, the central tetrahedron shares all 4 of its corner O atoms, the 2 face-centered tetrahedra share 2 of their corner O atoms, and the 4 edge-centered tetrahedra share just one of their O atoms with other SiO4 terahedra. This leaves a net average of 12 out of 24 total vertices for that portion of the 7 SiO4 tetrahedra which are considered to be a part of the unit cell for silica (see 3-D Unit Cell). SiO2 has a number of distinct crystalline forms (polymorphs) in addition to amorphous forms. With the exception of stishovite and fibrous silica, all of the crystalline forms involve tetrahedral SiO4 units linked together by shared vertices in different arrangements. Silicon-oxygen bond lengths vary between the different crystal forms, for example in α-quartz the bond length is 161 pm, whereas in α-tridymite it is in the range 154-171 pm. The Si-O-Si angle also varies between low values of 140° in α-tridymite, up to 180° in β-tridymite. In α-quartz the Si-O-Si angle is 144° [118]. [118] A.F. Holleman, E. Wiberg, Inorganic Chemistry, San Diego: Academic Press, (2001) |

The amorphous structure of glassy silica (SiO2) in two-dimensions.[...] Note that a fourth oxygen atom is bonded to each silicon atom, either behind the plane of the screen or in front of it; these atoms are omitted for clarity. [...] For example, in the unit cell of α-quartz, the central tetrahedron shares all 4 of its corner O atoms, the 2 face-centered tetrahedra share 2 of their corner O atoms, and the 4 edge-centered terahedra share just one of their O atoms with other SiO4 tetrahedra. This leaves a net average of 12 out of 24 total vertices for that portion of the 7 SiO4 tetrahedra which are considered to be a part of the unit cell for silica (see 3-D Unit Cell). SiO2 has a number of distinct crystalline forms (polymorphs) in addition to amorphous forms. With the exception of stishovite and fibrous silica, all of the crystalline forms involve tetrahedral SiO4 units linked together by shared vertices in different arrangements. Silicon-oxygen bond lengths vary between the different crystal forms, for example in α-quartz the bond length is 161 pm, whereas in α-tridymite it is in the range 154-171 pm. The Si-O-Si angle also varies between a low value of 140° in α-tridymite, up to 180° in β-tridymite. In α-quartz the Si-O-Si angle is 144°.[5] 5. Holleman, A. F.; Wiberg, E. (2001), Inorganic Chemistry, San Diego: Academic Press, ISBN 0-12-352651-5 |
The source is not mentioned. The copied passage starts on the previous page, where part of the figure caption has already been part of the main text. Also the reference to the literature has been taken from the source. |
|
| [32.] Mrs/Fragment 035 02 - Diskussion Bearbeitet: 16. May 2015, 11:13 Hindemith Erstellt: 29. December 2014, 08:43 (SleepyHollow02) | Fragment, Gesichtet, KomplettPlagiat, Mrs, SMWFragment, Schutzlevel sysop, Seiten, die magische ISBN-Links verwenden, Wikipedia Silicon dioxide 2011 |
|
|
| Untersuchte Arbeit: Seite: 35, Zeilen: 2-17 |
Quelle: Wikipedia Silicon dioxide 2011 Seite(n): 1 (online source), Zeilen: - |
|---|---|
| 3.4.2. Chemistry of silica
Silicon dioxide is formed when silicon is exposed to oxygen (or air). A very shallow layer (approximately 1 nm or 10 Å) of so-called native oxide is formed on the surface when silicon is exposed to air under ambient conditions. Higher temperatures and alternative environments are used to grow well-controlled layers of silicon dioxide on silicon, for example at temperatures between 600 and 1200 °C, using so-called dry or wet oxidation with O2 or H2O, respectively [119]. The depth of the layer of silicon replaced by the dioxide is 44% of the depth of the silicon dioxide layer produced [119]. Alternative methods used to deposit a layer of SiO2 include [120]
SiH4 + 2 O2 → SiO2 + 2 H2O.
Si(OC2H5)4 → SiO2 + 2 H2O + 4 C2H4.
Si(OC2H5)4 + 12 O2 → SiO2 + 10 H2O + 8 CO2.
[119] L. Sunggyu, Encyclopedia of chemical processing. CRC Press. (2006) [120] R. Doering, Y. Nishi (2007). Handbook of Semiconductor Manufacturing Technolog, Marcel Dekker, New York [121] A.B.D. Nandiyanto; S.-G Kim; F. Iskandar; and K. Okuyama (2009), Microporous and Mesoporous Materials 120 (3): 447–453 |
Chemistry
Silicon dioxide is formed when silicon is exposed to oxygen (or air). A very shallow layer (approximately 1 nm or 10 Å) of so-called native oxide is formed on the surface when silicon is exposed to air under ambient conditions. Higher temperatures and alternative environments are used to grow well-controlled layers of silicon dioxide on silicon, for example at temperatures between 600 and 1200 °C, using so-called dry or wet oxidation with O2 or H2O, respectively.[28] The depth of the layer of silicon replaced by the dioxide is 44% of the depth of the silicon dioxide layer produced.[28] Alternative methods used to deposit a layer of SiO2 include[29]
[28] Sunggyu Lee (2006). Encyclopedia of chemical processing. CRC Press. ISBN 0824755634. [29] Robert Doering, Yoshio Nishi (2007). Handbook of Semiconductor Manufacturing Technology. CRC Press. ISBN 1574446754. [30] A.B.D. Nandiyanto; S.-G Kim; F. Iskandar; and K. Okuyama (2009). "Synthesis of Silica Nanoparticles with Nanometer-Size Controllable Mesopores and Outer Diameters". Microporous and Mesoporous Materials 120 (3): 447–453. doi:10.1016/j.micromeso.2008.12.019. Innovation, Its Context and Tradition". |
The source is not mentioned. The references to the literature have also been taken from it. |
|
| [33.] Mrs/Fragment 035 18 - Diskussion Bearbeitet: 16. May 2015, 20:21 WiseWoman Erstellt: 16. May 2015, 16:51 (Hindemith) | Fragment, Gesichtet, KomplettPlagiat, Mrs, Ranjan 2008, SMWFragment, Schutzlevel sysop |
|
|
| Untersuchte Arbeit: Seite: 35, Zeilen: 18-23 |
Quelle: Ranjan 2008 Seite(n): 7, Zeilen: 3ff |
|---|---|
| 3.4.3. Silica nanoparticles
Among the numerous inorganic/organic hybrid materials, silica-polymer hybrid materials are one of the most commonly reported in the literature. This may be attributed to their wide use and the ease of particle synthesis. Silica nanoparticles have been used as fillers in the manufacture of paints, rubber products, and plastic binders [122]. Stöber and co-workers [123] reported a simple synthesis of monodisperse spherical silica particles. [112] H. Hommel, A. Touhami, A.P. Legrand, Makromol. Chem. 1993, 194 879 [122] N. Greenwood, A. Earnshaw, (1984), Chemistry of the Elements, Oxford: Pergamon, pp. 393–99 [123] W. Stöber, A. Fink, E.J. Bohn, J. Colloid Interface Sci. 1968, 26, 62. |
2.1. Silica nanoparticle
Among the numerous inorganic/organic hybrid materials, silica-polymer hybrid materials are one of the most commonly reported in the literature. This may be attributed to their wide use and the ease of particle synthesis. Silica nanoparticles have been used as fillers in the manufacture of paints, rubber products, and plastic binders.25 [...] [...] Stöber and co-workers32 reported a simple synthesis of monodisperse spherical silica particles. 25. Hommel, H.; Touhami,A.; Legrand, A. P. Makromol. Chem. 1993, 194 879. 32. Stöber, W.; Fink, A.; Bohn, E. J. J. Colloid Interface Sci. 1968, 26, 62. |
The source is not mentioned here. Possibly the reference "[122]" is a typo and should read "[112]." |
|
| [34.] Mrs/Fragment 036 19 - Diskussion Bearbeitet: 16. May 2015, 20:41 WiseWoman Erstellt: 28. March 2015, 13:04 (SleepyHollow02) | Fragment, Gesichtet, Mrs, SMWFragment, Schutzlevel sysop, Verschleierung, Wikipedia Fumed silica 2011 |
|
|
| Untersuchte Arbeit: Seite: 36, Zeilen: 19-23 |
Quelle: Wikipedia Fumed silica 2011 Seite(n): 1 (online source), Zeilen: - |
|---|---|
| Fumed silica
Fumed silica, also known as pyrogenic silica because it is produced in a flame by a vapor process at high temperature, consists of microscopic droplets of amorphous silica fused into branched, chainlike, three-dimensional secondary particles which then agglomerate into tertiary particles. |
Fumed silica
[...] Fumed silica, also known as pyrogenic silica because it is produced in a flame, consists of microscopic droplets of amorphous silica fused into branched, chainlike, three-dimensional secondary particles which then agglomerate into tertiary particles. |
The source is not mentioned. The passage is short and is purely descriptive. Nevertheless, the source should have been given. |
|
| [35.] Mrs/Fragment 037 13 - Diskussion Bearbeitet: 16. May 2015, 13:17 Hindemith Erstellt: 28. March 2015, 13:43 (SleepyHollow02) | Evonik 2009, Fragment, Gesichtet, Mrs, SMWFragment, Schutzlevel sysop, Verschleierung |
|
|
| Untersuchte Arbeit: Seite: 37, Zeilen: 13-20 |
Quelle: Evonik 2009 Seite(n): 4, Zeilen: l.col.: 19 ff. |
|---|---|
| Siloxane (ºSi-O-Siº) and silanol (ºSi-OH) groups are situated on the surface of fumed silica particles. This latter type (i.e. silanol) of functional groups in particular is responsible for the hydrophilic behavior of non-after treated fumed silica types. Figure 3.4 shows the surface groups of hydrophilic AEROSIL fumed silica. AEROSIL fumed silica can be surface-modified by reacting the silanol groups with suitable compounds such as silanes. For example Aerosil R972, is produced by reacting hydrophilic silica with dimethylchlorosilane as shown in Figure 3.5 .this product exhibits chemically bound dimethyl silyl groups on its surface and can no longer be wetted with water – in other words, it is hydrophobic. | Siloxane and silanol groups are situated on the surface of AEROSIL® particles. This latter type of functional group in particular is responsible for the hydrophilic behavior of non-aftertreated AEROSIL® types. Figure 1 shows the surface groups of hydrophilic AEROSIL® fumed silica. For basic details and applications of AEROSIL® fumed silica, see issue number 11 of the pigments publication series [1].
AEROSIL® fumed silica can be surface-modified by reacting the silanol groups with suitable compounds such as silanes. AEROSIL® R 972 is obtained by reaction with dimethyl-dichlorosilane, for example; this product exhibits chemically bound dimethyl silyl groups on its surface and can no longer be wetted with water – in other words, it is hydrophobic. (1) Technical Bulletin Fine Particles, No. 11, Basic Characteristics of AEROSIL® fumed silica, 6th edition 2003, Degussa AG, D-40402 Düsseldorf, Germany |
The source is not mentioned. |
|
| [36.] Mrs/Fragment 038 01 - Diskussion Bearbeitet: 16. May 2015, 13:09 Hindemith Erstellt: 28. March 2015, 13:51 (SleepyHollow02) | BauernOpfer, Evonik 2009, Fragment, Gesichtet, Mrs, SMWFragment, Schutzlevel sysop |
|
|
| Untersuchte Arbeit: Seite: 38, Zeilen: 1 ff. (entire page) |
Quelle: Evonik 2009 Seite(n): 4, Zeilen: r.col.: 1 ff. |
|---|---|

Figure 3.4 potential SiO2 surface groups of hydrophilic silicas [140] AEROSIL R 972 became the first hydrophobic silica to be manufactured on an industrial scale in 1962. Further hydrophobic AEROSIL grades are now available, produced by similar industrial-scale methods using corresponding silanes. Figure 3.5 provides a sampling of the many hydrophobic grades available and highlights the difference between them and hydrophilic AEROSIL grades according to the schematically represented surface groups. All hydrophobic AEROSIL types carry the suffix “R“ to indicate their water-repellent character. Figure 3.5 Hydrophobic Aerosil grade and their surface groups [140] [140] Technical Bulletin Fine Particles, No. 11, Basic Characteristics of AEROSILR fumed silica, 6th edition 2003, Degussa AG, D-40402 Dusseldorf,Germany |
Figure 1 Potential SiO 2 surface groups of hydrophilic silicas
AEROSIL® R 972 became the first hydrophobic silica to be manufactured on an industrial scale in 1962. Further hydrophobic AEROSIL® grades are now available, produced by similar industrial-scale methods using corresponding silanes. Figure 2 provides a sampling of the many hydrophobic grades available and highlights the difference between them and hydrophilic AEROSIL® grades according to the schematically represented surface groups. All hydrophobic AEROSIL® types carry the suffix „R“ to indicate their water-repellent character. Figure 2 Hydrophobic AEROSIL® grades and their surface groups |
The reference [140] might be a previous edition of the source documented here. The figures are therefore referenced appropriately, the text between the figures, however, is not referenced at all. |
|
| [37.] Mrs/Fragment 039 01 - Diskussion Bearbeitet: 16. May 2015, 13:15 Hindemith Erstellt: 28. March 2015, 13:58 (SleepyHollow02) | Evonik 2009, Fragment, Gesichtet, Mrs, SMWFragment, Schutzlevel sysop, Verschleierung |
|
|
| Untersuchte Arbeit: Seite: 39, Zeilen: 1-9 |
Quelle: Evonik 2009 Seite(n): 5, Zeilen: l.col.: 1 ff. |
|---|---|
| Hydrophilic and hydrophobic fumed silica grades have proven effective for numerous areas of application. In view of their use in the plastics industry, the principal properties of fumed silicas are listed below:
• Reinforcing fillers • Thickening and thixotropic agent for resins • Anti-sedimentation agent for cast resins and adhesives • Free flow aid for powder coatings [sic!] In contrast to silicas of mineral origin, meaning quartz powder which is used mainly as a “filler“ in the true sense of the word, the desired effects can be obtained by adding only relatively small amounts of synthetic silicas. |
Hydrophilic and hydrophobic AEROSIL® grades have proven effective for numerous areas of application. In view of their use in the plastics industry, the principal properties of fumed silicas are listed below:
• Reinforcing fillers • Thickening and thixotropic agent for resins • Anti-sedimentation agent for cast resins and adhesives • Free flow aid for powder coatings In contrast to silicas of mineral origin, meaning quartz powder which is used mainly as a „filler“ in the true sense of the word, the desired effects can be obtained by adding only relatively small amounts of synthetic silicas. |
The source is not mentioned. At the place marked with [sic!] an empty line is missing. Note that when copying and pasting from the source text, the empty line in the source text is omitted. |
|
| [38.] Mrs/Fragment 042 01 - Diskussion Bearbeitet: 16. May 2015, 20:36 WiseWoman Erstellt: 28. March 2015, 11:58 (SleepyHollow02) | Fragment, Gesichtet, Mrs, SMWFragment, Schutzlevel sysop, Seiten, die magische ISBN-Links verwenden, Verschleierung, Wikipedia Aluminium oxide 2010 |
|
|
| Untersuchte Arbeit: Seite: 42, Zeilen: 1-7 |
Quelle: Wikipedia Aluminium oxide 2010 Seite(n): 1 (online source), Zeilen: - |
|---|---|
| [The most common form of crystalline] alumina is known as corundum. The oxygen ions nearly form a hexagonal close-packed structure with aluminum ions filling two-thirds of the octahedral interstices. Each Al3+ center is octahedral. In terms of its crystallography, corundum adopts a trigonal Bravais lattice with a space group of R-3c (number 167 in the International Tables). The primitive cell contains two formula units of aluminium oxide. Alumina also exists in other phases, namely η-, χ-, γ-, δ-, and θ-aluminas. Each has a unique crystal structure and properties. The so-called β-alumina proved to be NaAl11O17. | The most common form of crystalline alumina is known as corundum. The oxygen ions nearly form a hexagonal close-packed structure with aluminium ions filling two-thirds of the octahedral interstices. Each Al3+ center is octahedral. In terms of its crystallography, corundum adopts a trigonal Bravais lattice with a space group of R-3c (number 167 in the International Tables). The primitive cell contains two formula units of aluminium oxide. Alumina also exists in other phases, namely γ-, δ-, η-, θ-, and χ-aluminas.[7] Each has a unique crystal structure and properties. The so-called β-alumina proved to be NaAl11O17.[8]
7. G. Paglia (2004). "Determination of the Structure of γ-Alumina using Empirical and First Principles Calculations Combined with Supporting Experiments" (free download). Curtin University of Technology, Perth. Retrieved 2009-05-05. 8. E. Wiberg and A. F. Holleman (2001). Inorganic Chemistry. Elsevier. ISBN 0-12-352651-5. |
The source is not mentioned. |
|
| [39.] Mrs/Fragment 042 09 - Diskussion Bearbeitet: 24. January 2015, 16:11 WiseWoman Erstellt: 28. December 2014, 08:06 (SleepyHollow02) | Fragment, Gesichtet, Mrs, SMWFragment, Schutzlevel sysop, Verschleierung, Wikipedia Cerium IV Oxide 2011 |
|
|
| Untersuchte Arbeit: Seite: 42, Zeilen: 9-14 |
Quelle: Wikipedia Cerium IV Oxide 2011 Seite(n): online, Zeilen: - |
|---|---|
| Cerium (IV) oxide, also known as ceric oxide, ceria, cerium oxide or cerium dioxide, is an oxide of the rare earth metal cerium. It is a pale yellow-white powder with the chemical formula CeO2. Cerium (IV) oxide is formed by the calcination of cerium oxalate or cerium hydroxide. Powdered ceria is slightly hygroscopic and will also absorb a small amount of carbon dioxide from the atmosphere [138]. Cerium also forms cerium (III) oxide, Ce2O3, but CeO2 is the most stable phase at room temperature and under atmospheric conditions.
[138] R. David Green. Part of PhD thesis CARBON DIOXIDE REDUCTION ON GADOLINIADOPED CERIA CA THODES. |
Cerium(IV) oxide, also known as ceric oxide, ceria, cerium oxide or cerium dioxide, is an oxide of the rare earth metal cerium. It is a pale yellow-white powder with the chemical formula CeO2.
Cerium(IV) oxide is formed by the calcination of cerium oxalate or cerium hydroxide. Powdered ceria is slightly hygroscopic and will also absorb a small amount of carbon dioxide from the atmosphere.[2] Cerium also forms cerium(III) oxide, Ce2O3, but CeO2 is the most stable phase at room temperature and under atmospheric conditions. 2. ROBERT DAVID GREEN. CARBON DIOXIDE REDUCTION ON GADOLINIA-DOPED CERIA CA THODES. Submitted in partial fulfillment of the requirements for the degree of Doctor of Philosophy. Dissertation Adviser: Dr. Chung-Chiun Liu. Department of Chemical Engineering CASE WESTERN RESERVE UNIVERSITY. May, 2009: http://www.google.dk/url?sa=t&source=web&cd=2&ved=0CBwQhgIwAQ&url=http%3A%2F%2Fetd.ohiolink.edu%2Fsend-pdf.cgi%2FGreen%2520Robert%2520David.pdf%3Fcase1232574534&ei=pWOcTILyGs-lOLHsma0M&usg=AFQjCNEqEjilE7V7obSPlyWV0AJAuP4Uog&sig2=2gq3rN4kF9YQ5yEN67A0eQ |
This small paragraph seems to be taken verbatim from WP 2011, reference included. ROBERT DAVID GREEN's Thesis is the only reference in Mrs' reference list of more than 300 which is spelt in capital letters (as in WP) and includes the space in the word "Cathodes". Mrs apparently is not aware that "partial fulfillment of the requirements for the degree" is a complete PhD Thesis (the rest is coursework) and thus translates this to "Part of a PhD Thesis" |
|
| [40.] Mrs/Fragment 048 26 - Diskussion Bearbeitet: 19. April 2015, 18:50 WiseWoman Erstellt: 27. December 2014, 21:42 (Hindemith) | Fornes 2003, Fragment, Gesichtet, Mrs, SMWFragment, Schutzlevel sysop, Verschleierung |
|
|
| Untersuchte Arbeit: Seite: 48, Zeilen: 26-34 |
Quelle: Fornes 2003 Seite(n): 252, 254, Zeilen: 252: 13 ff.; 254: 1 ff. |
|---|---|
| Some characteristic properties of the α- and γ-forms of polyamide 6 are listed in Table 4.1 [173- 187]. The heats of fusion, ΔHf°, are the values reported by Illers [184]. It should be noted that other values for the α-form have been reported in the literature [180, 184, 186, 188-191] and Wunderlich has suggested a value of 230 J/g based on a compromise of various reports in the literature [192]. The effect of crystallization temperature and time on the formation of the α- versus γ-forms has been widely studied [185, 193, 194]. Gurato and his coworkers have shown that crystallization for extended periods of time below ~130 °C leads only to the γ-crystallites while above ~190 °C only the α-form is produced. Temperatures in between these limits result in a mixture of the two forms, with higher fractions of α-form being [produced at higher temperatures.]
[173] S.M: Aharoni, n-Polyamides [sic], their synthesis, structure, and properties. Chichester; New York: Wiley; 1997. [174] Arimoto H, Ishibashi M, Hirai M, Chatani Y. Journal of Polymer Science Partt A 1965; 3(1):317–26. [175] D.R. Holmes, C.W. Bunn, D.J. Smith, Journal of Polymer Science 1955;17:159–77. [176] N. Murthy, Polymer Communications 1991;32(10):301–5. [177] F. Rybnikar, Burda J. Faserforsch u Textiltech 1961;12:324–31. [178] L.G. Roldan, H.S. Kaufman, Polymer Letter 1960;1:603–8. [179] T. Itoh, H. Miyaji, K. Asai, Japanese Journal of Applied Physics 1975;14(2):206–15. [180] A. Reichle, A. Prietzschk, Angew Chem 1962;74:562–9. [181] L.G. Wallner, Monatsh 1948;79:279–95. [182] K.H. Illers, H. Haberkorn, P. Simak, Makromolekulare Chemie1972;158: 285–311. [183] K.H. Illers, Makromolekulare Chemie 1978;179(2):497–507. [184] K.H. Illers, H. Haberkorn, Makromolekulare Chemie 1971;142:31–67. [185] S. Gogolewski, M. Gasiorek, K. Czerniawska, A. Pennings. Colloid and Polymer Science 1982; 260(9):859–63. [186] D.C. Vogelsong, J Polymer Science 1963; 1(Pt. A):1055–68. [187] P. Marx, C. Smith, A. Worthington, M. Dole, Journal of Physical Chemistry; 59: 1015–9. [188] M. Dole, B. Wunderlich, Makromolekulare Chemie 1959;34:29–49. [189] M. Inoue, J Polymer Science Part A 1963; 1:2013–20. [190] J.R. Starkweather, P. Zoller, G.A. Jones, J Polymer Science, Polymer Physics Edition 1984; 22(9):1615–21. [191] B. Wunderlich, Macromolecular physics, vol. 3. New York: Academic Press; 1973. [192] G. Gurato, A. Fichera, F.Z. Grandi, R. Zannetti, P. Canal, Makromolekulare Chemie 1974;175(3):953–75. [193] M. Kyotani, S. Mitsuhashi, J Polymer Science, Part A-2 1972; 10(8): 1497–508. [194] R.J. [sic] Brill für Praktische Chemie 1942;161:49–64. |
Table 9.1 lists characteristic properties of the α- and γ-forms of nylon 6 [6-20]. The heats of fusion, ΔHf°, are the values reported by Illers [17]. It should be noted that other values for the α-form have been reported in the literature [13, 17, 19, 21-25] and Wunderlich has suggested a value of 230 J/g based on a compromise of various reports in the literature [26].
[...] The effect of crystallization temperature and time on the formation of the α versus γ-forms has been widely studied. For example, three independent investigations [18, 27, 28], have shown that crystallization for extend periods of [page 254] time below ~130°C leads solely to the γ-crystallites while above ~190°C only the α-form is produced. Temperatures in between these limits result in a mixture of the two forms, with higher fractions of α produced at higher temperatures. [...] [29] 6. Aharoni, SM, n-Nylons, their synthesis, structure, and properties. New York: J. Wiley & Sons. 1997. 7. Arimoto, H, Ishibashi, M, Hirai, M, Chatani, Y. J Polymer Sci, Part A 1965;3(1): 317-26. 8. Holmes, DR, Bunn, CW, Smith, DJ. J Polymer Sci 1955;17: 159-77. 9. Kohen, MI, ed. Nylon plastics handbook. Hanser: New York. 1995. 10. Murthy, NS. Polym Commun 1991;32(10): 301-5. 11. Rybnikar, F, Burda, J. Faserforsch u Textiltech 1961;12: 324-31. 12. Roldan, LG, Kaufman, HS. Polym Letts 1960;1: 603-8. 13. Itoh, T, Miyaji, H, Asai, K. Jpn J Appl Phys 1975;14(2): 206-15. 14. Reichle, A, Prietzschk, A. Angew Chem 1962;74: 562-9. 15. Wallner, LG. Monatsh 1948;79: 279-95. 16. Illers, KH, Haberkorn, H, Simak, P. Makromol Chem 1972;158: 285-311. 17. Illers, KH. Makromol Chem 1978;179(2): 497-507. 18. Illers, KH, Haberkorn, H. Makromol Chem 1971;142: 31-67. 19. Gogolewski, S, Gasiorek, M, Czerniawska, K, Pennings, AJ. Colloid Polym Sci 1982;260(9): 859-63. 20. Vogelsong, DC. J Polym Sci 1963;1(Pt. A): 1055-68. 21. Rybnikar, F. Chem listy 1958;52: 1042-8. 22. Marx, P, Smith, CW, Worthington, AE, Dole, M. J Phys Chem 1955;59: 1015-9. 23. Dole, M, Wunderlich, B. Makromol Chem 1959;34: 29-49. 24. Inoue, M. J Polymer Sci Pt A 1963;1: 2013-20. 25. Starkweather, HW, Jr., Zoller, P, Jones, GA. J Polym Sci, Part B 1984;22(9): 1615-21. 26. Wunderlich, B, Macromolecular physics (vol 3). New York: Academic Press. 1973. 27. Gurato, G, Fichera, A, Grandi, FZ, Zannetti, R, Canal, P. Makromol Chem 1974;175(3): 953-75. 28. Kyotani, M, Mitsuhashi, S. J Polym Sci, Part A-2 1972;10(8): 1497-508. 29. Brill, R. J prakt Chem 1942;161: 49-64. |
The source is not mentioned. Note that most references are -- compared to the source -- moved by one. |
|
| [41.] Mrs/Fragment 049 01 - Diskussion Bearbeitet: 30. March 2015, 11:55 SleepyHollow02 Erstellt: 27. December 2014, 16:59 (Hindemith) | Fornes 2003, Fragment, Gesichtet, Mrs, SMWFragment, Schutzlevel sysop, Verschleierung |
|
|
| Untersuchte Arbeit: Seite: 49, Zeilen: 1 ff. (entire page) |
Quelle: Fornes 2003 Seite(n): 254, 255, 256, 258, 259, Zeilen: 254: 3 ff.; 255: 1 ff.; 256: 1 ff.; 258: 1 ff.; 259: 1 ff. |
|---|---|
| The disappearance of the γ-form at ~190 °C has been commonly referred to as the Brill transition, a phenomenon first observed in polyamide 66 [195]. This transition corresponds to the merging of the two reflections of the α-form into a single peak in plots of X-ray diffraction intensity versus 2 [196-199] as the sample is heated. Kyotani and Mitsuhashi [194] also found that very short crystallization times at either 100 or 200 °C lead to both crystalline forms, while longer times produced predominantly γ- and α-form, respectively. In general, rapid cooling or quenching from the melt produces the γ-form [196, 200-202]. Annealing also affects the crystal structure. For example, annealing of quenched samples or those crystallized between 100 and 150 °C, at 200 °C for extended times leads to the conversion of the γ- into α-form [194]. Gogolewki et al. [186] and Gurato et al. [193] demonstrated similar annealing results. Based on the above, it is clear that, in general, rapid cooling and low temperature crystallization promotes the γ-form of polyamide 6, while higher crystallization temperatures or slow cooling leads to the α-form. Kyotani and Mitsuhashi [194] attribute the temperature dependence to the crystallization rates of the two forms; i.e. at temperatures below 130 °C the rate of formation of γ is faster, while above ~190 °C the crystallization rate of α-form is faster, while at intermediate temperatures, the rates are comparable. Interestingly, the maximum crystallization rate of polyamide 6 occurs at approximately 140 °C [203]. The temperature dependence of the crystallization rate above this maximum is dominated by the driving force for crystallization, i.e. the degree of undercooling, while below, the rate is dominated by the resistance to crystallization, i.e. polymer chain mobility. Therefore, it may be postulated that conditions of limited polymer chain mobility favor the crystallization of the γ-form for polyamide 6.
Studies of polyamide 6-based nanocomposites have caused renewed interest in the factors affecting the crystalline structure. As mentioned above, Kojima et al. [203] and Liu et al. [165] reported that when the one nanometer thick platelets of montmorillonite are dispersed in polyamide 6 the polymer crystallizes in the γ-form. Wu and coworkers [204-207] examined the influence of thermal treatment on the crystallization behavior of melt processed nanocomposites using FTIR, WAXD, and DSC. From FTIR measurements on thin film samples, it has been claimed that the hydrogen bonding in both crystalline forms of polyamide 6 is weakened by the presence of the clay. WAXD analysis revealed that nanocomposites films exhibit both crystalline forms when slowly cooled from the melt; whereas, pure polyamide 6 gave predominantly the α-form. Methods that permit higher cooling rates, e.g. air and water-cooling, have increased the content of the γ-phase especially for the nanocomposite. Further WAXD investigations conducted on samples that had been annealed at different temperatures showed the onset of [the γ- to α- transition, normally observed around 130 °C for p ure polyamide 6, in this case at 120 °C, occurred approximately 40 °C higher for the nan ocomposite.] [165] L.M. Liu, Z.N. Qi, X.G. Zhu, J Applied Polymer Science 1999, 71, 1133. [186] D.C. Vogelsong, J Polymer Science 1963; 1(Pt. A):1055–68. [193] M. Kyotani, S. Mitsuhashi, J Polymer Science, Part A-2 1972; 10(8): 1497–508. [194] R.J. Brill für Praktische Chemie 1942;161:49–64. [195] N. Murthy, S.M. Aharoni, A. Szollosi, J Polymer Science, Polymer Physics Edition 1985; 23(12):2549–65. [196] N.Murthy, S.A. Curran, S.M. Aharoni, H. Minor, Macromolecules 1991; 24(11):3215–20. [197] C. Ramesh, E.B. Gowd, Macromolecules 2001; 34(10):3308–13. [198] D.M. Lincoln, R.A. Vaia, Z.G. Wang, B.S. Hsiao, R. Krishnamoorti, Polymer 2001; 42(25):09975–85. [199] D.R. Salem, R. Moore, H. Weigmann, J. Polymer Science, Part B: Polymer Physics 1987; 25(3):567–89. [200] I. Campoy, M.A. Gomez, C. Marco, Polymer 1998; 39(25):6279–88. [201] A. Okada, M. Kawasumi, I. Tajima, J Applied Polymer Science 1989;37(5):1363–71. [202] J.H. Magill, Polymer 1962; 3:655–64. [203] Y. Kojima, A. Usuki, M. Kawasumi, A. Okada, J Material Research 1993; 8(5):1185–9. [204] Q. Wu, X. Liu, L. Berglund, Macromolecular Rapid Communications 2001;22(17): 1438– 40. [205] X.Liu, Q. Wu, European Polymer Journal 2002;38(7):1383–9. [206] Q. Wu, X. Liu, Berglund LA. Polymer 2002;43(8):2445–9. [207] X. Liu, Q. Wu, Polymer 2002;43(6):1933–6. |
The disappearance of the γ−form at ~190°C has been commonly referred to as the Brill transition, a phenomenon first observed in nylon 6,6 [29]. This transition corresponds to the merging of the two reflections of the α-form into a single peak in plots of X-ray diffraction intensity versus 2θ [30-33] as the sample is heated.
[page 255] Kyotani and Mitsuhashi [28] found that very short crystallization times at either 100 and 200°C lead to both crystalline forms, while longer times produced predominantly γ and α , respectively. In general rapid cooling or quenching from the melt produces the γ-form [30, 34-36]. Annealing also affects the crystalline structure. For example, annealing of quenched samples, or those crystallized between 100 and 150 °C, at 200 °C for extended times leads to the conversion of the γ into α [28]. Similar annealing results were demonstrated by Gogolewki et al. [19] and Gurato et al. [27]. [...] Based on the above, it is clear that, in general, rapid cooling and low temperature crystallization promotes the γ-form of nylon 6, while higher crystallization temperatures or slow cooling leads to the α-form. Kyotani and Mitsuhashi [28] attribute the temperature dependence of the α- and γ-form to their crystallization rates; i.e., at temperatures below 130°C the rate of formation of γ is faster, while above ~190°C the crystallization rate of α is faster, while at intermediate temperatures, the rates are comparable. [page 256] Interestingly, the maximization crystallization rate of nylon 6 occurs at approximately 140°C [37]. The temperature dependence of the crystallization rate above this maximum is dominated by the driving force for crystallization, i.e., the degree of undercooling, while below, the rate is dominated by the resistance to crystallization, i.e., polymer chain mobility. Therefore, it may be postulated that conditions of limited polymer chain mobility favor the crystallization of the γ-form for nylon 6. [page 258] Studies of nylon 6-based nanocomposites have caused renewed interest in the factors affecting the crystalline structure. As mentioned above, Kojima et al. [1] and Liu et al. [2] reported that when the one nanometer thick platelets of montmorillonite are dispersed in nylon 6 the polymer crystallizes in the γ-form. [...] Wu and coworkers [49-52] examined the influence of thermal treatment on the crystallization behavior of melt processed nanocomposites using FTIR, WAXD, and DSC. From FTIR measurements on thin film samples, it has been claimed that the hydrogen bonding in both crystalline forms of nylon 6 are weakened by the presence of the clay. WAXD analysis revealed that nanocomposites films exhibit both crystalline forms when slowly cooled from the melt; whereas, pure nylon 6 gave predominantly the α form. Methods that permit higher cooling rates, e.g., air and water-cooling, increased the content of the γ- phase especially for the nanocomposite. Further WAXD investigations conducted on samples that had been annealed at different temperatures showed the onset of [page 259] the γ to α transition, normally observed around 130°C for pure nylon 6, in this case 120°C, occurred approximately 40°C higher for the nanocomposite. 1. Kojima, Y, Usuki, A, Kawasumi, M, Okada, A, Fukushima, Y, Kurauchi, T, Kamigaito, O. J Mater Res 1993;8(5): 1185-9. 2. Liu, L, Qi, Z, Zhu, X. J Appl Polym Sci 1999;71(7): 1133-8. 19. Gogolewski, S, Gasiorek, M, Czerniawska, K, Pennings, AJ. Colloid Polym Sci 1982;260(9): 859-63. 27. Gurato, G, Fichera, A, Grandi, FZ, Zannetti, R, Canal, P. Makromol Chem 1974;175(3): 953-75. 28. Kyotani, M, Mitsuhashi, S. J Polym Sci, Part A-2 1972;10(8): 1497-508. 29. Brill, R. J prakt Chem 1942;161: 49-64. 30. Murthy, NS, Aharoni, SM, Szollosi, AB. J Polym Sci, Part B 1985;23(12): 2549-65. 31. Murthy, NS, Curran, SA, Aharoni, SM, Minor, H. Macromolecules 1991;24(11): 3215-20. 32. Ramesh, C, Gowd, EB. Macromolecules 2001;34(10): 3308-13. 33. Lincoln, DM, Vaia, RA, Wang, ZG, Hsiao, BS, Krishnamoorti, R. Polymer 2001;42(25): 9975-85. 34. Salem, DR, Moore, RAF, Weigmann, HD. J Polym Sci, Part B 1987;25(3): 567-89. 35. Campoy, I, Gomez, MA, Marco, C. Polymer 1998;39(25): 6279-88. 36. Okada, A, Kawasumi, M, Tajima, I, Kurauchi, T, Kamigaito, O. J Appl Polym Sci 1989;37(5): 1363-71. 37. Magill, JH. Polymer 1962;3: 655-64. 49. Wu, Q, Liu, X, Berglund, LA. Macromolecular Rapid Comm 2001;22(17): 1438-40. 50. Liu, X, Wu, Q. European Polymer Journal 2002;38(7): 1383-9. 51. Wu, Q, Liu, X, Berglund, LA. Polymer 2002;43(8): 2445-9. |
The source is not mentioned. |
|
| [42.] Mrs/Fragment 050 01 - Diskussion Bearbeitet: 19. April 2015, 18:52 WiseWoman Erstellt: 28. December 2014, 00:34 (Hindemith) | BauernOpfer, Fornes 2003, Fragment, Gesichtet, Mrs, SMWFragment, Schutzlevel sysop |
|
|
| Untersuchte Arbeit: Seite: 50, Zeilen: 1 ff. (entire page) |
Quelle: Fornes 2003 Seite(n): 254, 258, 259, Zeilen: 254: 1 ff.; 258: last lines; 259: 1 ff. |
|---|---|
| [Further WAXD investigations conducted on samples that had been annealed at different temperatures showed the onset of] the γ- to α- transition, normally observed around 130 °C for p ure polyamide 6, in this case at 120 °C, occurred approximately 40 °C higher for the nan ocomposite. Interestingly, an unexpected higher amount of γ-form was observed after annealing at 200 °C than a t 180 °C. Lastly, non-isothermal crystallization surprisingly showed that the degree of crystallinity increased with increasing cooling rate for the nanocomposites, in contrast to what is observed in pure polyamide 6 and other polymers.
Table 4.1 Miscellaneous data for the α- and γ-crystalline forms of polyamide 6 a Hexagonal lattice constants were calculated based on the monoclinic parameters a=0.933nm, b=1.688nm, and c=0.478nm and β=121°. The hexagonal lattice constant, a, was taken as the average of a and half of b of the monoclinic unit cell. The constant c representing the fiber axis remained fixed [161]. [161] T.D. Fornes, D.R. Paul, Polymer 2003, 44, 3945. [173] S.M: Aharoni, n-Polyamides, their synthesis, structure, and properties. Chichester; New York: Wiley; 1997. [174] Arimoto H, Ishibashi M, Hirai M, Chatani Y. Journal of Polymer Science Partt A 1965; 3(1):317–26. [175] D.R. Holmes, C.W. Bunn, D.J. Smith, Journal of Polymer Science 1955;17:159–77. [176] N. Murthy, Polymer Communications 1991;32(10):301–5. [177] F. Rybnikar, Burda J. Faserforsch u Textiltech 1961;12:324–31. [178] L.G. Roldan, H.S. Kaufman, Polymer Letter 1960;1:603–8. [179] T. Itoh, H. Miyaji, K. Asai, Japanese Journal of Applied Physics 1975;14(2):206–15. [180] A. Reichle, A. Prietzschk, Angew Chem 1962;74:562–9. [181] L.G. Wallner, Monatsh 1948;79:279–95. [182] K.H. Illers, H. Haberkorn, P. Simak, Makromolekulare Chemie1972;158: 285–311. [183] K.H. Illers, Makromolekulare Chemie 1978;179(2):497–507. [184] K.H. Illers, H. Haberkorn, Makromolekulare Chemie 1971;142:31–67. [185] S. Gogolewski, M. Gasiorek, K. Czerniawska, A. Pennings. Colloid and Polymer Science 1982; 260(9):859–63. [186] D.C. Vogelsong, J Polymer Science 1963; 1(Pt. A):1055–68. |
Table 9.1 Miscellaneous data for the α and γ crystalline forms of nylon 6.
(a) Hexagonal lattice constants were calculated based on the monoclinic parameters a = 0.933 nm, b = 1.688nm, and c = 0.478nm and β = 121°. The hexagonal lattice constant, a, was taken as the average of a and half of b of the monoclinic unit cell. The constant c representing the fiber axis remained fixed. [page 258] Further WAXD investigations conducted on samples that had been annealed at different temperatures showed the onset of [page 259] the γ to α transition, normally observed around 130°C for pure nylon 6, in this case 120°C, occurred approximately 40°C higher for the nanocomposite. Interestingly, an unexpected higher amount of γ-form was observed after annealing at 200°C than at 180°C. Lastly, non-isothermal crystallization surprisingly showed that the degree of crystallinity increased with increasing cooling rate for the nanocomposites, counter to what is observed in pure nylon 6 and other polymers. 6. Aharoni, SM, n-Nylons, their synthesis, structure, and properties. New York: J. Wiley & Sons. 1997. 7. Arimoto, H, Ishibashi, M, Hirai, M, Chatani, Y. J Polymer Sci, Part A 1965;3(1): 317-26. 8. Holmes, DR, Bunn, CW, Smith, DJ. J Polymer Sci 1955;17: 159-77. 9. Kohen, MI, ed. Nylon plastics handbook. Hanser: New York. 1995. 11. Rybnikar, F, Burda, J. Faserforsch u Textiltech 1961;12: 324-31. 12. Roldan, LG, Kaufman, HS. Polym Letts 1960;1: 603-8. 13. Itoh, T, Miyaji, H, Asai, K. Jpn J Appl Phys 1975;14(2): 206-15. 14. Reichle, A, Prietzschk, A. Angew Chem 1962;74: 562-9. 15. Wallner, LG. Monatsh 1948;79: 279-95. 16. Illers, KH, Haberkorn, H, Simak, P. Makromol Chem 1972;158: 285-311. 17. Illers, KH. Makromol Chem 1978;179(2): 497-507. 18. Illers, KH, Haberkorn, H. Makromol Chem 1971;142: 31-67. 19. Gogolewski, S, Gasiorek, M, Czerniawska, K, Pennings, AJ. Colloid Polym Sci 1982;260(9): 859-63. 20. Vogelsong, DC. J Polym Sci 1963;1(Pt. A): 1055-68. |
The source is not mentioned. Possibly the table can also be found in the reference [161] (still to be checked), but even if this was the case, this reference would not make clear that the entire page is taken from the source. |
|
| [43.] Mrs/Fragment 051 02 - Diskussion Bearbeitet: 22. March 2015, 15:21 WiseWoman Erstellt: 27. December 2014, 16:14 (Hindemith) | Fornes 2003, Fragment, Gesichtet, Mrs, SMWFragment, Schutzlevel sysop, Verschleierung |
|
|
| Untersuchte Arbeit: Seite: 51, Zeilen: 2-10 |
Quelle: Fornes 2003 Seite(n): 259, Zeilen: 10ff |
|---|---|
| Wu and Liao examined the effect of thermal history and filler concentration on the crystal structure of polyamide 6 nanocomposites formed by in situ polymerization using synthetic saponite and natural montmorillonite [208, 209]. Similar crystalline behavior was observed for the pure and nanocomposite materials when samples were slowly or rapidly cooled from the melt; however, nanocomposites containing a low concentration of saponite, i.e. 2.5 wt. %, favored the formation of the α-form regardless of the cooling conditions, whereas by increasing silica content γ-form is more dominant (Figure 4.5). Annealing studies showed that higher amounts of γ were present in the nanocomposite than in pure polyamide 6 after annealing at high temperatures, except at low saponite contents.
[208] T.M. Wu, C.S. Liao, Macromolecular Chemistry and Physics 2000;201(18):2820–5. [209] T.M. Wu, E.C. Chen, C.S. Liao, Polymer Engineering Science 2002;42(6):1141–50. |
Wu and Liao examined the effect of thermal history and filler concentration on the crystal structure of nylon 6 nanocomposites formed by in situ polymerization using synthetic saponite and natural montmorillonite [53, 54]. Similar crystalline behavior was observed for the pure and nanocomposite materials when samples were slowly or rapidly cooled from the melt; however, nanocomposites containing a low concentration of saponite, i.e., 2.5 weight %, favored the formation of the α-form regardless of the cooling conditions. Annealing studies showed that higher amounts of γ were present in the nanocomposite than in pure nylon 6 after annealing at high temperatures, except at low saponite contents.
53. Wu, T-M, Liao, C-S. Macromolecular Chem Phys 2000;201(18): 2820-5. 54. Wu, T-M, Chen, E-C, Liao, C-S. Polym Engng Sci 2002;42(6): 1141-50. |
A source is not mentioned. The description from Fornes' thesis is also to be found at Fornes, T.D. & Paul, D. R. (2003). Crystallization behavior of nylon 6 nanocomposites. In Polymer 44, pp. 3945–3961. |
|
| [44.] Mrs/Fragment 054 04 - Diskussion Bearbeitet: 22. March 2015, 13:49 WiseWoman Erstellt: 27. December 2014, 16:10 (Hindemith) | Fornes 2003, Fragment, Gesichtet, Mrs, SMWFragment, Schutzlevel sysop, Verschleierung |
|
|
| Untersuchte Arbeit: Seite: 54, Zeilen: 4-12 |
Quelle: Fornes 2003 Seite(n): 259, 260, Zeilen: 259: last paragraph; 260: 1ff |
|---|---|
| Some studies have explored the differences between polyamide 6 nanocomposites formed by in situ polymerization and by melt processing. Based on DSC and solid state NMR studies, VanderHart et al. [214, 215] concluded that the silicate layers promote the γ-form regardless of the formation technique, and that γ-crystallites reside near the polyamide/clay interface. Lincoln et al. [164] used small angle X-ray scattering, in addition to WAXD and DSC, to infer about the crystalline morphology of these two types of nanocomposites. They concluded that the clay platelets disrupt the formation of crystallites and the extent of platelet-crystallite interactions is dependent upon the technique used to form the nanocomposites. In other words, the nature of the bond between the clay and polyamide chains seems to influence clay-crystallite interactions.
[164] D. Lincoln, R. Vaia, Z. Wang, Hsiao, B. S.; Krishnamoorti, R. Polymer 2001, 42, 9975. [214] D.L. VanderHart, A. Asano, J.W. Gilman, Chemistry of Materials 2001; 13(10): 3796–809. [215] D.L. VanderHart, A. Asano, J.W. Gilman, Chemistry of Materials 2001; 13(10): 3781–95. |
Some studies have explored the differences between nylon 6 nanocomposites formed by in situ polymerization and by melt processing. Based on DSC and solid state NMR studies, VanderHart et al. [55, 56] concluded that the silicate layers promote the γ-form regardless of the formation technique, and
[page 260] that γ-crystallites reside near the polyamide/clay interface. Lincoln et al. [57] used small angle X-ray scattering, in addition to WAXD and DSC, to infer about the crystalline morphology of these two types of nanocomposites. They concluded that the clay platelets disrupt the formation of crystallites and the extent of platelet-crystallite interactions is dependent upon the technique used to form the nanocomposites. In other words, the nature of the bond between the clay and polyamide chains seems to influence clay-crystallite interactions. 55. VanderHart, DL, Asano, A, Gilman, JW. Chem Maters 2001;13(10): 3796-809. 56. VanderHart, DL, Asano, A, Gilman, JW. Chem Maters 2001;13(10): 3781-95. 57. Lincoln, DM, Vaia, RA, Wang, Z-G, Hsiao, BS. Polymer 2001;42(4): 1621-31. |
A source is not mentioned. The description from Fornes' thesis is also to be found at Fornes, T.D. & Paul, D. R. (2003). Crystallization behavior of nylon 6 nanocomposites. In Polymer 44, pp. 3945–3961. |
|
| [45.] Mrs/Fragment 058 04 - Diskussion Bearbeitet: 24. January 2015, 22:49 WiseWoman Erstellt: 28. December 2014, 09:08 (SleepyHollow02) | Fragment, Gesichtet, Journal of Nanomaterials cfp 2009, Mrs, SMWFragment, Schutzlevel sysop, Verschleierung |
|
|
| Untersuchte Arbeit: Seite: 58, Zeilen: 4-15 |
Quelle: Journal of Nanomaterials cfp 2009 Seite(n): online, Zeilen: - |
|---|---|
| In the general research area of polymer nanocomposites, a number of critical issues need to be addressed before the full potential of polymer nanocomposites can actually be realized. While a number of advances have recently been made in the area of polymer nanocomposites, the studies on understanding of the effects of processing parameters on the structure, morphology, and functional properties of polymer nanocomposites are essential. There is a need for characterization techniques to quantify the concentration and distributions of nanoparticles as well as to assess the strength at the interface between the polymer and nanoparticles. Also, there is a need for the development of better models which are able to predict the mechanical properties of the polymer nanocomposites as a function of many factors, including nanoparticle orientation, the type of functional groups, and the molecular weight of polymer matrix. The relationship between the structural distributions of the nanoparticles and the ultimate properties of the polymer nanocomposites also needs to be elucidated. | In the general research area of polymer nanocomposites, a number of critical issues need to be addressed before the full potential of polymer nanocomposites can actually be realized. While a number of advances have recently been made in the area of polymer nanocomposites, the studies on understanding of the effects of processing parameters on the structure, morphology, and functional properties of polymer nanocomposites are deficient. There is a need for characterization techniques to quantify the concentration and distributions of nanoparticles as well as to assess the strength at the interface between the polymer and nanoparticles. Also, there is a need for the development of better models able to predict the mechanical properties of the polymer nanocomposites as functions of myriad factors including nanoparticle orientation, the type of functional groups, and the molecular weight of polymer chain. The relationships between the structural distributions and the ultimate properties of the polymer nanocomposites also need to be elucidated. |
Minor editing done, no source is given. |
|
| [46.] Mrs/Fragment 061 06 - Diskussion Bearbeitet: 2. May 2015, 20:43 WiseWoman Erstellt: 24. January 2015, 19:02 (Klgn) | Bhattacharya et al. 2008, Fragment, Gesichtet, Mrs, SMWFragment, Schutzlevel sysop, Verschleierung |
|
|
| Untersuchte Arbeit: Seite: 61, Zeilen: 6-13 |
Quelle: Bhattacharya et al. 2008 Seite(n): 299, Zeilen: 11-17 |
|---|---|
| Electron microscopy is a process of obtaining images using electrons and is frequently used when the magnification required is much larger than what can be achieved by light microscopes that means; when the particles to be monitored are smaller than the wavelength of the visual light (< 400 nm). The physical effect behind this principle is based on wave-particle duality of electrons. The emitted electrons are high-energy matter having wavelength much smaller than that of light and this allows for the resolution of smaller objects. Moreover, the electrons interact with samples in various ways and this allows for the determination of detailed information about them. | Electron microscopy is a process of obtaining images using electrons and is frequently used when the magnification required is much larger than what can be achieved by light microscopes, i.e., the particles to be monitored are smaller than the wavelength of the visual light (< 400 nm). It is based on wave-particle duality of electrons. The emitted electrons are high-energy matter having wavelengths much smaller than that of light and this allows for the resolution of smaller objects. Moreover, the electrons interact with samples in various ways and this allows for the determination of detailed information about them. |
|
|
| [47.] Mrs/Fragment 061 13 - Diskussion Bearbeitet: 18. May 2015, 20:31 WiseWoman Erstellt: 4. January 2015, 00:08 (WiseWoman) | Fragment, Gesichtet, Mrs, SMWFragment, Schutzlevel sysop, Verschleierung, Wikipedia Electron Microscope 2011 |
|
|
| Untersuchte Arbeit: Seite: 61, Zeilen: 13-19 |
Quelle: Wikipedia Electron Microscope 2011 Seite(n): 1 (online source), Zeilen: - |
|---|---|
| The advantages of electron microscopy over X-ray crystallography are, that the specimen need not be a single crystal or even a polycrystalline powder, and also that the Fourier transform reconstruction of the object's magnified structure occurs physically and thus avoids the need for solving the phase problem faced by the X-ray crystallographers after obtaining their X-ray diffraction patterns of a single crystal or polycrystalline powder. The major disadvantage of the transmission electron is the need for extremely thin sections of the specimens, typically less than 100 nanometers. | The advantages of electron microscopy over X-ray crystallography are that the specimen need not be a single crystal or even a polycrystalline powder, and also that the Fourier transform reconstruction of the object's magnified structure occurs physically and thus avoids the need for solving the phase problem faced by the X-ray crystallographers after obtaining their X-ray diffraction patterns of a single crystal or polycrystalline powder. The major disadvantage of the transmission electron microscope is the need for extremely thin sections of the specimens, typically about 100 nanometers. |
No source is given. |
|
| [48.] Mrs/Fragment 061 20 - Diskussion Bearbeitet: 18. May 2015, 20:35 WiseWoman Erstellt: 24. January 2015, 19:23 (Klgn) | Bhattacharya et al. 2008, Fragment, Gesichtet, KomplettPlagiat, Mrs, SMWFragment, Schutzlevel sysop |
|
|
| Untersuchte Arbeit: Seite: 61, Zeilen: 20-21 |
Quelle: Bhattacharya et al. 2008 Seite(n): 299, Zeilen: 20-22 |
|---|---|
| The two main electron microscopic techniques available for nanocomposite imaging are the scanning electron microscopy (SEM) and transmission electron microscopy (TEM). | The two main electron microscopic techniques available for nanocomposite imaging are the scanning electron microscopy (SEM) and transmission electron microscopy (TEM). |
"Spliced" plagiarism: The fragment Mrs/Fragment 061 06 is from the same source as this sentence, followed by a portion from the Wikipedia in Mrs/Fragment 061 13, then this fragment, followed by another portion from the Wikipedia Mrs/Fragment 061 23. |
|
| [49.] Mrs/Fragment 061 23 - Diskussion Bearbeitet: 24. January 2015, 23:11 WiseWoman Erstellt: 28. December 2014, 09:48 (SleepyHollow02) | Fragment, Gesichtet, KomplettPlagiat, Mrs, SMWFragment, Schutzlevel sysop, Wikipedia Scanning electron microscope 2011 |
|
|
| Untersuchte Arbeit: Seite: 61, Zeilen: 23-26 |
Quelle: Wikipedia Scanning electron microscope 2011 Seite(n): online, Zeilen: - |
|---|---|
| A scanning electron microscope (SEM) is a type of electron microscope that images a sample by scanning it with a high-energy beam of electrons in a raster scan pattern. The electrons interact with the atoms that make up the sample producing signals that contain information about the sample's surface topography, composition, and other properties such as electrical conductivity. | A scanning electron microscope (SEM) is a type of electron microscope that images a sample by scanning it with a high-energy beam of electrons in a raster scan pattern. The electrons interact with the atoms that make up the sample producing signals that contain information about the sample's surface topography, composition, and other properties such as electrical conductivity. |
No source is given. |
|
| [50.] Mrs/Fragment 062 00 - Diskussion Bearbeitet: 16. May 2015, 20:00 WiseWoman Erstellt: 16. May 2015, 11:36 (Hindemith) | Cruz 2007, Fragment, Gesichtet, KomplettPlagiat, Mrs, SMWFragment, Schutzlevel sysop |
|
|
| Untersuchte Arbeit: Seite: 62, Zeilen: Figure 5.2 |
Quelle: Cruz 2007 Seite(n): 1 (online source), Zeilen: - |
|---|---|

Figure 5.2 Comparison of the light microscope with TEM and SEM. |

Fig. 2: Principal features of a light microscope, a transmission electron microscope (TEM), and a scanning electron microscope (SEM), drawn to emphasize the similarities of overall design. [...] |
No source for the figure is given. |
|
| [51.] Mrs/Fragment 062 02 - Diskussion Bearbeitet: 24. January 2015, 23:17 WiseWoman Erstellt: 28. December 2014, 10:02 (SleepyHollow02) | Fragment, Gesichtet, KomplettPlagiat, Mrs, SMWFragment, Schutzlevel sysop, Wikipedia Transmission electron microscopy 2011 |
|
|
| Untersuchte Arbeit: Seite: 62, Zeilen: 2-6 |
Quelle: Wikipedia Transmission electron microscopy 2011 Seite(n): online, Zeilen: - |
|---|---|
| Transmission electron microscopy (TEM) is a microscopy technique whereby a beam of electrons is transmitted through an ultra thin specimen, interacting with the specimen as it passes through. An image is formed from the interaction of the electrons transmitted through the specimen; the image is magnified and focused onto an imaging device, such as a fluorescent screen, on a layer of photographic film, or to be detected by a sensor such as a CCD camera. | Transmission electron microscopy (TEM) is a microscopy technique whereby a beam of electrons is transmitted through an ultra thin specimen, interacting with the specimen as it passes through. An image is formed from the interaction of the electrons transmitted through the specimen; the image is magnified and focused onto an imaging device, such as a fluorescent screen, on a layer of photographic film, or to be detected by a sensor such as a CCD camera. |
No source is given. |
|
| [52.] Mrs/Fragment 063 01 - Diskussion Bearbeitet: 18. May 2015, 20:37 WiseWoman Erstellt: 24. January 2015, 20:26 (Klgn) | Bhattacharya et al. 2008, Fragment, Gesichtet, Mrs, SMWFragment, Schutzlevel sysop, Verschleierung |
|
|
| Untersuchte Arbeit: Seite: 63, Zeilen: 1-17 |
Quelle: Bhattacharya et al. 2008 Seite(n): 307; 308, Zeilen: 307: 9 ff.; 308: 1 ff. |
|---|---|
| 5.4. Spectroscopic technique [240, 241]
Spectral techniques are generally used to probe the chemical make-up of macromolecular materials such as functional groups, structural conformation and component concentration. The main spectral techniques applied in polymer nanocomposite research are Fourier transform infra-red spectroscopy (FTIR), nuclear magnetic resonance (NMR) and ultraviolet (UV) spectroscopy. [Figure] Figure 5.3 EM Spectrum showing the range of frequencies and wavelength of radiation. Spectral techniques involve the interaction of molecules of a specimen or sample with electromagnetic (EM) radiation. It essentially monitors changes in energy states of molecules in response to EM radiation. Figure 5.3 shows the EM spectrum and the corresponding wavelength of the various radiations. Important is to understand the energy state of molecules when EM radiation is observed. Basically, when an atom or a molecule absorbs energy, it proceeds from the initial or ground state to a higher or excited state. These energy states are said to be quantumized and a particular value exists for each state. This could be related to the “spinning” of the sub-atomic particles, vibration of the chemical bonds, and so on. Fourier transform infra-red spectroscopy (FTIR) FTIR is a technique that utilizes the vibration response of molecules when exposed to infrared (IR) radiation. |
[page 307]
6.4 Spectroscopic Techniques Spectral techniques are generally used to probe the chemical make-up of macromolecular materials, such as functional groups, structural conformation, and component concentrations. The main spectral techniques applied in polymer nanocomposite research are Fourier [page 308] transform infra-red spectroscopy (FTIR), nuclear magnetic resonance (NMR), and ultraviolet (UV) spectroscopy. This section will outline these methods and review some studies from literature. [Figure] Figure 6.46: EM spectrum showing the range of frequencies and wavelength of radiation. The shaded region is that of visible light Spectral techniques involve the interaction of molecules of a specimen or sample with electromagnetic (EM) radiation. It essentially monitors changes in energy states of molecules in response to EM radiation. Figure 6.46 shows the EM spectrum and the corresponding wavelengths of the various radiations. Of particular importance is to understand the energy “states” of molecules when EM radiation is absorbed. Basically, when an atom or molecule absorbs energy, it proceeds from the initial or ground state to a higher or excited state. These energy states are said to be quantized and a particular value exists for each state. This could be related to the “spinning” of the nucleus, vibration of the bonds, and so on. [...] 6.4.1 Fourier Transform Infra-Red (FTIR) Spectroscopy FTIR is a technique that utilizes the vibrational response of molecules when exposed to infrared (IR) radiation. |
The text and the figures are from different sources: The text is from Bhattacharya et al. 2008, the figures in the dissertation are edited versions of figures from the Wikimedia Commons http://commons.wikimedia.org/wiki/File:EM_spectrum.svg?uselang=en. There is no source for either the text or the figures given. |
|
| [53.] Mrs/Fragment 064 03 - Diskussion Bearbeitet: 16. May 2015, 19:17 WiseWoman Erstellt: 25. January 2015, 09:10 (Klgn) | Fragment, Gesichtet, Gupta 1997, Mrs, SMWFragment, Schutzlevel sysop, Verschleierung |
|
|
| Untersuchte Arbeit: Seite: 64, Zeilen: 3-7 |
Quelle: Gupta 1997 Seite(n): 238, Zeilen: 15-17 |
|---|---|
| 5.5. Thermal characterization [242-244]
Thermoanalytical techniques are used for characterization of glass transition and melting temperature, thermal stability and other properties as a function of temperature of polymers and fibers. [242] A. Turi, Thermal characterization of polymeric materials / ed. by Edith 2 London : Academic Press, 1997 [243] Höhne, Günther, Differential scanning calorimetry : with 19 tables., Berlin : Springer, 2003 [244] E. A. Turi, thermal analysis of polymers, Academic press, New York1982 [sic] |
10.4 THERMAL CHARACTERIZATION
Thermoanalytical techniques are used for characterization of glass transition and melting temperatures, thermal stability and other properties as a function of temperature of polymers and fibres [22]. 22. Turi, E.A. (1982) Thermal Analysis of Polymers, Academic Press, New York |
The source is not mentioned. |
|
| [54.] Mrs/Fragment 064 07 - Diskussion Bearbeitet: 17. May 2015, 09:05 WiseWoman Erstellt: 25. January 2015, 08:55 (Klgn) | Bhattacharya et al. 2008, Fragment, Gesichtet, Mrs, SMWFragment, Schutzlevel sysop, Verschleierung |
|
|
| Untersuchte Arbeit: Seite: 64, Zeilen: 7 ff. |
Quelle: Bhattacharya et al. 2008 Seite(n): 320, Zeilen: 3-17 |
|---|---|
| The main thermal techniques are differential scanning calorimetry (DSC), thermal gravimetric analysis (TGA), dynamic mechanical analysis (DMA) and heat distortion temperature (HDT). Thermal analysis is based on the detection of changes in the heat content (enthalpy) or the specific heat of a sample with temperature.
5.5.1. Differential Scanning Calorimetry (DSC) Differential scanning calorimetry is a thermo-analytical method that measures the difference between the amount of heat necessary to raise the temperature of sample and reference. It is used to study thermal transitions of a polymer. As thermal energy is supplied to the sample, its enthalpy increase and the temperature rises by an amount determined by the specific heat of the sample. The specific heat of the material changes slowly with temperature in a particular physical state, but alter sharply or discontinuously when a change of the state of the matter takes place. Apart from increasing the sample temperature, the supply of thermal energy may also induce physical or chemical changes in the sample (e.g. melting or decomposition) accompanied by a change in enthalpy in the form of the latent heat of fusion, heat of reaction, or others. Such enthalpy changes may be detected by thermal analysis and can be related to the processes occurring in the sample. |
The main thermal techniques are differential scanning calorimetry (DSC), thermal gravimetric analysis (TGA), dynamic mechanical analyzer (DMA), heat distortion temperatures (HDT), and cone calorimetry. Thermal analysis is based on the detection of changes in the heat content (enthalpy) or the specific heat of a sample with temperature.
6.7.1 Differential Scanning Calorimetry (DSC) DSC is a technique which is part of a group of techniques called thermal analysis (TA). As thermal energy is supplied to the sample, its enthalpy increases and the temperature rises by an amount determined by the specific heat of the sample. The specific heat of a material changes slowly with temperature in a particular physical state, but alters sharply or discontinuously when a change of state takes place. Apart from increasing the sample temperature, the supply of thermal energy may also induce physical or chemical changes in the sample (e.g., melting or decomposition) accompanied by a change in enthalpy in the form of the latent heat of fusion, heat of reaction, or others. Such enthalpy changes may be detected by thermal analysis and can be related to the processes occurring in the sample. |
No source is given. |
|
| [55.] Mrs/Fragment 065 01 - Diskussion Bearbeitet: 16. May 2015, 12:19 Hindemith Erstellt: 23. March 2015, 12:48 (SleepyHollow02) | Fragment, Gesichtet, Gill et al 2010, Mrs, SMWFragment, Schutzlevel sysop, Verschleierung |
|
|
| Untersuchte Arbeit: Seite: 65, Zeilen: 1 ff. |
Quelle: Gill et al 2010 Seite(n): 167, Zeilen: l.col. 22 ff. |
|---|---|
| Based on mechanism of operation DSC can be classified into two types.
1. Heat flux DSC 2. Power Compensated DSC Heat Flux DSC In a heat flux DSC, the sample material, enclosed in a pan and an empty reference pan placed on a thermoelectric disk surrounded by a furnace. The furnace is heated at a linear heating rate and the heat is transferred to the sample and reference pan through thermoelectric disk. However owing to the heat capacity of the sample there exists a temperature difference between the sample and reference pans which is measured by area thermocouples and the consequent heat flow is determined by the thermal equivalent of Ohm’s law, q=ΔT / R Where q is sample heat flow, ΔT is temperature difference between sample and reference and R is resistance of thermoelectric disk. |
Based on the mechanism of operation, DSCs can be classified into two types: heat-flux DSCs and power-compensated DSCs.11 In a heat flux DSC, the sample material, enclosed in a pan, and an empty reference pan are placed on a thermoelectric disk surrounded by a furnace.11,12 The furnace is heated at a linear heating rate, and the heat is transferred to the sample and reference pan through the thermoelectric disk.11,12 However, owing to the heat capacity (Cp) of the sample, there would be a temperature difference between the sample and reference pans, which is measured by area thermocouples, and the consequent heat flow is determined by the thermal equivalent of Ohm’s law:
q =ΔT/R, where q is “sample heat flow”, ΔT is “temperature difference between sample and reference”, and R is “resistance of thermoelectric disk”.12 11. Haines PJ, Reading, M, Wilburn FW. Differential thermal analysis and differential scanning calorimetry. In Brown ME (ed): Handbook of Thermal Analysis and Calorimetry, vol 1. The Netherlands: Elsevier Science BV, 1998;279 –361. 12. Danley RL. New heat flux DSC measurement technique. Thermochim Acta 2002;395:201–208. |
The source is not mentioned. |
|
| [56.] Mrs/Fragment 066 01 - Diskussion Bearbeitet: 16. May 2015, 12:21 Hindemith Erstellt: 23. March 2015, 12:57 (SleepyHollow02) | Fragment, Gesichtet, Gill et al 2010, Mrs, SMWFragment, Schutzlevel sysop, Verschleierung |
|
|
| Untersuchte Arbeit: Seite: 66, Zeilen: 1-4 |
Quelle: Gill et al 2010 Seite(n): 167, Zeilen: r.col.: 11 ff. |
|---|---|
| Power Compensated DSC
In power compensated calorimeters, the sample and reference pan are in separate furnaces heated by separate heaters. Both the sample and reference are maintained at the same temperature and the difference in thermal power required to maintain them at the same temperature is measured and plotted as a function of temperature or time. |
In a power-compensated DSC, the sample and reference pans are placed in separate furnaces heated by separate heaters.11,13 The sample and reference are maintained at the same temperature, and the difference in thermal power required to maintain them at the same temperature is measured and plotted as a function of temperature or time.11
11. Haines PJ, Reading, M, Wilburn FW. Differential thermal analysis and differential scanning calorimetry. In Brown ME (ed): Handbook of Thermal Analysis and Calorimetry, vol 1. The Netherlands: Elsevier Science BV, 1998;279 –361. 13. Zucca N, Erriu G, Onnis S, Longoni A. An analytical expression of the output of a power-compensated DSC in a wide temperature range. Thermochim Acta 2002;143:117–125. |
The source is not mentioned. The copied text starts on the previous page. |
|
| [57.] Mrs/Fragment 067 05 - Diskussion Bearbeitet: 16. May 2015, 19:25 WiseWoman Erstellt: 24. March 2015, 13:53 (SleepyHollow02) | Fragment, Gesichtet, Mrs, Nairn 2007, SMWFragment, Schutzlevel sysop, Verschleierung |
|
|
| Untersuchte Arbeit: Seite: 67, Zeilen: 5-12 |
Quelle: Nairn 2007 Seite(n): 7, Zeilen: 4ff |
|---|---|
| Polymer molecular weight is important because it determines many physical properties. Some examples include the temperatures for transitions from liquids to waxes, to rubbers, to solids, and mechanical properties such as stiffness, tensile strength and elongation at break of fibers, as well as viscoelasticity, toughness, and viscosity. If molecular weight is too low, the transition temperatures and the mechanical properties will generally be too low for the polymer material to have any useful commercial applications. For a polymer to be useful it must have transition temperatures to waxes or liquids that are above room temperatures and it must have mechanical properties sufficient to bear design loads. | Polymer molecular weight is important because it determines many physical properties. Some examples include the temperatures for transitions from liquids to waxes to rubbers to solids and mechanical properties such as stiffness, strength, viscoelasticity, toughness, and viscosity. If molecular weight is too low, the transition temperatures and the mechanical properties will generally be too low for the polymer material to have any useful commercial applications. For a polymer to be useful it must have transition temperatures to waxes or liquids that are above room temperature (i.e., be a solid at room temperature) and it must have mechanical properties sufficient to bear design loads. |
The source is not mentioned. The passage can also be found in [2], a source from 2003 (PDF file properties), which, however, is difficult to cite. Note that the title of the chapter at the beginning of the page comes with the references "[245-248]", which however do not indicate that parts of the chapter are taken verbatim from somewhere else. [245] Collins, Edward A., Experiments in polymer science, Wiley, c 1973 [246] Billmeyer, Fred W., Textbook of polymer science,- 3. Ed, Wiley, 1984 [247] Harry. R. Allcock, F.W. Lampe, Contemporary Polymer Chemistry, Prentice-Hall 1981 [248] Kulicke, Werner-Michael. , Analysis of polymers : molar mass and molar mass distribution of polymers, polyelectrolytes and latices;, Basel : Hüthig & Wepf, 1992 |
|
| [58.] Mrs/Fragment 070 04 - Diskussion Bearbeitet: 19. April 2015, 19:11 WiseWoman Erstellt: 28. December 2014, 02:33 (Hindemith) | Fragment, Gesichtet, Gupta 1997, Mrs, SMWFragment, Schutzlevel sysop, Verschleierung |
|
|
| Untersuchte Arbeit: Seite: 70, Zeilen: 4-18 |
Quelle: Gupta 1997 Seite(n): 214, Zeilen: 11 ff. |
|---|---|
| Polymer solutions show very high viscosity which varies not only with concentration but also with molecular weight. This property of polymers has been used as a method of determining the molecular weight of polymers. A parameter called intrinsic viscosity [η] is strongly dependent on the molecular dimensions of the solute particles. Since molecular dimensions depend on molecular weight, suitable calibration curves have been developed which led to well-known relation called the Mark-Houwink equation:
Where k and a are constants and M is the molecular weight of the polymer. M may represent Mn or Mw, depending on the molecular weight average used in the calibration curve. The intrinsic viscosity [η] is defined as follow: Where η and η0 are the viscosities of the solution and the solvent, respectively, and c is the concentration. The last expression on the right-hand side is simply to define the symbol ηsp (specific viscosity) in subsequent discussion. The intrinsic viscosity is therefore determined by plotting ηsp/c against c and extrapolating the plot to zero concentration, as shown in Figure 5.5 |
Polymer solutions show very high viscosity which varies not only with concentration but also with molecular weight. This property of polymers has been used as a method of determining the molecular weight of polymers.
A parameter called 'intrinsic viscosity' (also called 'limiting viscosity number' in modern nomenclature), denoted with brackets as [η], is strongly dependent on the molecular dimensions of the solute particles. Since molecular dimensions depend on molecular weight, suitable calibration curves have been developed which lead to a well-known relation called the Mark-Houwink equation: where K and a are constants and M is the molecular weight of the polymer. M may represent Mn or Mw, depending on the molecular weight average used in the calibration curve. The intrinsic viscosity [η] is defined as follows: where η and η0 are the viscosities of the solution and the solvent, respectively, and c is the concentration. The last expression on the right-hand side is simply to define the symbol ηsp (specific viscosity) in subsequent discussion. The intrinsic viscosity is therefore determined by plotting ηsp/c against c and extrapolating the plot to zero concentration, as shown in Fig. 10.8. |
The source is not mentioned. |
|
| [59.] Mrs/Fragment 071 01 - Diskussion Bearbeitet: 16. May 2015, 17:07 Hindemith Erstellt: 28. December 2014, 20:41 (Hindemith) | Fragment, Gesichtet, Mrs, Russo 2008, SMWFragment, Schutzlevel sysop, Verschleierung |
|
|
| Untersuchte Arbeit: Seite: 71, Zeilen: 1-3 |
Quelle: Russo 2008 Seite(n): 1, Zeilen: 15 ff. |
|---|---|
| The Ubbelohde capillary viscometer
The most useful kind of viscometer for determining intrinsic viscosity is the "suspended level" or Ubbelohde viscometer, sketched below: |
The Ubbelohde capillary viscometer
The most useful kind of viscometer for determining intrinsic viscosity is the "suspended level" or Ubbelohde viscometer, sketched below: |
The source is not mentioned. To be continued on the next page. The sketches of the viscometer are not identical. A source for the sketch could be [3]. |
|
| [60.] Mrs/Fragment 072 01 - Diskussion Bearbeitet: 25. March 2015, 11:07 SleepyHollow02 Erstellt: 28. December 2014, 18:36 (Hindemith) | Fragment, Gesichtet, KomplettPlagiat, Mrs, Russo 2008, SMWFragment, Schutzlevel sysop |
|
|
| Untersuchte Arbeit: Seite: 72, Zeilen: 1 ff. (entire page) |
Quelle: Russo 2008 Seite(n): 1, 2, 9, Zeilen: 1: 18 ff.; 2: 1 ff.; 9: 1 ff. |
|---|---|
| The viscometer is called "suspended level" because the liquid initially drawn into the small upper bulb is not connected to the reservoir as it flows down the capillary during measurement. The capillary is suspended above the reservoir. In conjunction with the pressure-equalization tube, this ensures that the only pressure difference between the top of the bulb and the bottom of the capillary is that due to the hydrostatic pressure i.e., the weight of the liquid. Other designs, e.g., the Cannon-Fenske viscometer, do not provide for this, and will give erroneous results in an intrinsic viscosity determination. Such viscometers are useful in other experiments--e.g., checking the stability of some polymer solution, where one is only interested in measuring a change in the flow time.
Basic Relations for Capillary Viscometry Here is presented the basic relation of capillary viscometry, which is known as Poiseulle's law [250]. Where: · Q is the volumetric flow rate through the capillary in cm3/s; · P is the pressure head forcing the liquid through the capillary (usually, just the hydrostatic pressure of the liquid itself); · R is the radius of the capillary; · l is the length of the capillary; and, · η is the viscosity The bulb volume in the Ubellohde viscometer is fixed. Thus, the flow rate, Q, is just inversely proportional to the time between marks. Since P is usually the hydrostatic pressure, which is proportional to the density of the fluid, we have: η ∝ tρ This simple relationship is the "ideal gas law" of capillary [sic] [250] S.F. Sun, Physical chemistry of macromolecules: basic principles and issues, 2nd edition - Hoboken, NJ : Wiley, 2004 |
The viscometer is called "suspended level" because the liquid initially drawn into the small upper bulb is not connected to the reservoir as it flows down the capillary during measurement. The capillary is suspended above the reservoir. In conjunction with the pressure-equalization tube, this ensures that the only pressure difference between the top of the bulb and the bottom of the capillary is that due to the hydrostatic pressure--i.e., the weight of the liquid. Other designs, e.g., the Cannon-Fenske viscometer, do not provide for this, and will give erroneous results in an intrinsic viscosity determination. Such
[page 2] viscometers are useful in other experiments--e.g., checking the stability of some polymer solution, where one is only interested in measuring a change in the flow time. [page 9] Appendix 1. Basic Relations for Capillary Viscometry Here is presented the basic relation of capillary viscometry, which is known as Poiseulle's law. [...] where: • Q is the volumetric flow rate through the capillary in cm3/s; • P is the pressure head forcing the liquid through the capillary (usually, just the hydrostatic pressure of the liquid itself); • R is the radius of the capillary; • l is the length of the capillary; and, • η is the viscosity [...] The bulb volume in the Ubellohde viscometer is fixed. Thus, the flow rate, Q, is just inversely proportional to the time between marks. Since P is usually the hydrostatic pressure, which is proportional to the density of the fluid, we have: η ∝ tρ This simple relationship is the "ideal gas law" of capillary viscosity (i.e., you should remember it!). |
The source is not given. The reference [250] does contain Poiseulle's law, but not the text parallels. |
|
| [61.] Mrs/Fragment 073 01 - Diskussion Bearbeitet: 19. April 2015, 19:18 WiseWoman Erstellt: 28. December 2014, 19:47 (Hindemith) | Fragment, Gesichtet, Mrs, Russo 2008, SMWFragment, Schutzlevel sysop, Verschleierung |
|
|
| Untersuchte Arbeit: Seite: 73, Zeilen: 1 ff. (entire page) |
Quelle: Russo 2008 Seite(n): 2, Zeilen: 3 ff. |
|---|---|
| Use of the Ubbelohde viscometer
Capillary viscometry is conceptually simple: the time it takes a volume of polymer solution to flow through a thin capillary is compared to the time for a solvent flow. It turns out that the flow time for either is proportional to the viscosity, and inversely proportional to the density The relative viscosity is defined to be the ratio ηsol'n /ηsolvent . For most polymer solutions at the concentrations of interest, ρsol'n / ρsolvent ࣈ 1 . Thus, to a very good approximation, the relative viscosity is a simple time ratio: "specific viscosity" is also defined to be the fractional change in viscosity upon addition of polymer: Both ηrel and ηsp depend on the polymer concentration, so to extract the "intrinsic" properties of the polymer chain itself, one must extrapolate to zero concentration. Measuring at zero concentration (c=0) would be useless, but this concept of extrapolating to c=0 is very important in polymer characterization and in thermodynamics generally. The two quantities that are commonly plotted versus concentration and extrapolated to c=0 are ηsp and c-1ln (ηrel). A typical plot is Figure 5.5. |
Use of the Ubbelohde viscometer
[...] Capillary viscometry is conceptually simple: the time it takes a volume of polymer solution to flow through a thin capillary is compared to the time for a solvent flow. It turns out that the flow time for either is proportional to the viscosity, and inversely proportional to the density. We define the relative viscosity to be the ratio ηsol'n /ηsolvent. For most polymer solutions at the concentrations of interest, ρsol'n / ρsolvent ࣈ 1. Thus, to a very good approximation, the relative viscosity is a simple time ratio: We also define a "specific viscosity" to be the fractional change in viscosity upon addition of polymer: Both ηrel and ηsp depend on the polymer concentration, so to extract the "intrinsic" properties of the polymer chain itself, one must extrapolate to zero concentration. Measuring at zero concentration (c=0) would be useless, but this concept of extrapolating to c=0 is very important in polymer characterization and in thermodynamics generally. The two quantities that are commonly plotted vs. concentration and extrapolated to c=0 are ηsp and c-1ln (ηrel). A typical plot is shown below |
The source is not mentioned. |
|
| [62.] Mrs/Fragment 074 01 - Diskussion Bearbeitet: 19. April 2015, 19:09 WiseWoman Erstellt: 28. December 2014, 02:28 (Hindemith) | Fragment, Gesichtet, Gupta 1997, Mrs, SMWFragment, Schutzlevel sysop, Verschleierung |
|
|
| Untersuchte Arbeit: Seite: 74, Zeilen: 1 ff. (entire page) |
Quelle: Gupta 1997 Seite(n): 215, 216, Zeilen: 215: last paragraph; 216: 1 ff. |
|---|---|
| 5.6.3. Gel permeation chromatography (GPC)
Gel permeation chromatography (GPC) is quite useful for routine estimate of molecular weight, owing to its convenience and the possibility of simultaneous evaluation of various averages of molecular weight, thus providing information about the molecular weight distribution. The GPC technique is based on the separation of solute molecules according to their size by passing the polymer solution through a column packed with microporous gel particles. A known small volume of polymer solution is injected into an already stabilized current of the solvent through the column, and then the out flowing liquid from the column is analyzed for the concentration of the solute as a function of time. Separations of molecules occur by their preferential penetration into the pores of the gel filled in the column, depending on their sizes. Small molecules penetrate more easily than the larger one, while the very large ones may either partially penetrate or not penetrate at all. Thus, during the passage of the solution through the column the largest molecules will take the shortest time while the smallest one will take the longest time to elute from the column. The eluting liquid passing through the detector is measured for its solute concentration through its refractive index or optical density. The detector signal, which is proportional to the solute concentration, thus gives a trace as a function of time or elution volume, as shown in Figure 5.6 for a polydisperse polymer sample. Figure 5.6 Typical GPC curve for a polydisperse polymer sample. |
(e) Gel permeation chromatography
Gel permeation chromatography (GPC) is quite useful for routine estimate of molecular weight, owing to its convenience and the possibility of simultaneous evaluation of various averages of molecular weight, thus providing information about the molecular weight distribution. [page 216] Fig. 10.9 Typical GPC curve for a polydisperse polymer sample. The GPC technique is based on the separation of solute molecules according to their sizes by passing the polymer solution through a column packed with microporous gel particles. A known small volume of polymer solution is injected into an already stabilized current of the solvent through the column, and then the out-flowing liquid from the column is analysed for the concentration of the solute as a function of time. Separation of molecules occurs by their preferential penetration into the pores of the gel filled in the column, depending on their sizes. Small molecules penetrate more easily than the larger ones, while the very large ones may either partially penetrate or not penetrate at all. Thus, during the passage of the solution through the column the largest molecules will take the shortest time while the smallest ones will take the longest time to elute from the column. The eluting liquid passing through a detector is measured for its solute concentration through its refractive index or optical density. The detector signal, which is proportional to the solute concentration, thus gives a trace as a function of time or elution volume, as shown in Fig. 10.9 for a polydisperse polymer sample. |
The source is not mentioned. The two figures are similar, but not identical. |
|
| [63.] Mrs/Fragment 075 01 - Diskussion Bearbeitet: 19. April 2015, 19:16 WiseWoman Erstellt: 28. December 2014, 16:45 (Hindemith) | BauernOpfer, Fragment, Gesichtet, Kothari 1997, Mrs, SMWFragment, Schutzlevel sysop |
|
|
| Untersuchte Arbeit: Seite: 75, Zeilen: 1 ff. (entire page) |
Quelle: Kothari 1997 Seite(n): 251 ff., Zeilen: 251: last lines; 252: 1 ff. |
|---|---|
| 5.7. Tensile properties
Tensile properties of textile materials are measured using machines designed to impart, or transmit, force/extension to the material and measure the response of the material to the applied action. Tensile testing machine for textile materials are classified according to their operating principle as (1) Constant rate of extension (CRE), (2) Constant rate of traverse (CRT), (3) Constant rate of loading (CRL). Most fibers and filament yarn testers employ constant rate of extension (CRE) as the straining principle because there are difficulties in extension measurement on constant rate of loading (CRL) testers due to creep and the accuracy achieved using constant rate of traverse (CRT) testers is much lower than that achieved with other types of tester. The load-elongation characteristic curve obtained on CRE testers provide important information about the relationship between force and elongation during the time up to the fiber rupture and can be uses to determine a number of important tensile test parameters [44]. The following parameters are determined with reference to tensile tester equipment: - Breaking load- the peak load that is reached during a tensile test (units: N, cN, mN). - Elongation at break – the elongation at breaking load expressed as percentage of the original length (unit: %). - Tenacity – the breaking load per unit linear density of the unstrained specimen (units: Ntex-1, cN tex-1, cN dtex-1). - Modulus of elasticity or Young’s modulus – the slope of the stress-strain curve in the elastic region between the origin and the yield point. (units: Ntex-1, cN tex-1, cN dtex-1). - Work of rupture – the work done from the point of pretensional load to the point of breaking load. The energy required to bring a specimen to the breaking load can be obtained from the area under the load-elongation curve. Work of rupture is dependent on the linear density and length of specimen (units: N m, cN m) [44] V.B.Gupta and V.K.Kothart , Manufactured fiber technology, 1997 Chapman and Hall |
11.4 TENSILE PROPERTIES
Tensile properties of textile materials are measured using machines designed to impart, or transmit, force/extension to the material and measure the response of the material to the applied action. Tensile testing machines for textile materials are classiFied according to their operating principle as follows [3]. Type Principle of operation CRE Constant rate of extension CRT Constant rate of traverse (pendulum type) CRL Constant rate of loading (inclined plane type) [page 252] Most fibre and filament yam testers employ constant rate of extension (CRE) as the straining principle because there are difficulties in extension measurement on constant rate of loading (CRL) testers due to creep and the accuracy achieved using constant rate of traverse (CRT) testers is much lower than that achieved with other types of tester. The load-elongation characteristic curves (LE characteristic curves) obtained on CRE testers provide important information about the relationship between force and elongation during the time up to the fibre/yarn rupture and can be used to determine a number of important tensile test parameters. 11.4.1 TERMS AND DEFINITIONS Figure 11.1 shows a typical load-elongation curve for a fibre. The following parameters are determined with reference to this curve: • Breaking load (or force) - the peak load (or force) that is reached during a tensile test (units: N, cN, mN). [...] • Breaking extension - the elongation at breaking load expressed as a percentage of the original length (unit: %). [page 253] • Tenacity - the breaking load per unit linear density of the unstrained specimen (units: N tex-1, cN tex-1, cN dtex-1). [...] • Modulus of elasticity or Young's modulus - the slope of the stress-strain curve in the elastic region between the origin and the yield point. It is calculated from the slope of the load-elongation curve at the midpoint of the elongation in the elastic region (units: Ntex-1, cNtex-1, cN dtex-1). • Work of rupture - the work done from the point of pretensional load to the point of breaking load. The energy required to bring a specimen to the breaking load can be obtained from the area under the load- elongation curve. Work of rupture is dependent on the linear density and length of the specimen (units: N m, cN cm). [...] |
The source is mentioned, but it does not become clear that the entire page is taken from it. |
|
| [64.] Mrs/Fragment 081 01 - Diskussion Bearbeitet: 16. May 2015, 20:14 WiseWoman Erstellt: 16. May 2015, 16:14 (Hindemith) | Ahn et al 2004, Fragment, Gesichtet, Mrs, SMWFragment, Schutzlevel sysop, Verschleierung |
|
|
| Untersuchte Arbeit: Seite: 81, Zeilen: 1-6 |
Quelle: Ahn et al 2004 Seite(n): 816, Zeilen: r.col: 10ff |
|---|---|
| Im et al. [259] have reported that when silica nanoparticles were used as filler, they acted as a lubricant rather than degrading the polymer matrix when exposed to the high shear forces and heat experienced during melt compounding. This is because the silica particles are spherical and have a smooth, non-porous surface, which lowers the coefficient of friction. The above properties allow for the possibility of improving the processibility, although decreasing the melt viscosity, have a direct effect on physical property of fibers.
[259] S. S. Im, S. C. Chung, W. G. Hahm, and S. G. Oh, Macromol. Res., 10, 221 (2002). |
Im et al.7 reported that when silica nanoparticles were used as a filler, they acted as a lubricant rather than degrading the polymer matrix when exposed to the high shear force and heat experienced during melt compounding. This is because the silica particles have a spherical shape and smooth, nonporous surfaces, which lower the coefficient of friction.
The above properties not only allow for the possibility of improving the processability but also promise additional applications for nanoparticle-filled polymer composites. 7. Im, S. S.; Chung, S. C.; Hahm, W. G.; Oh, S. G. Macromol Res 2002, 10, 221. |
Note that the Seung-Soon Im is not the first author of the cited paper, he is the third author. This mistake can be found also in the source. Note also that the referenced paper Chung et al. (2002) does not contain the parallel text. |
|
| [65.] Mrs/Fragment 082 05 - Diskussion Bearbeitet: 2. May 2015, 21:07 WiseWoman Erstellt: 25. January 2015, 09:30 (Klgn) | Bhattacharya et al. 2008, Fragment, Gesichtet, Mrs, SMWFragment, Schutzlevel sysop, Verschleierung |
|
|
| Untersuchte Arbeit: Seite: 82, Zeilen: 5-9 |
Quelle: Bhattacharya et al. 2008 Seite(n): 120, Zeilen: 3-8 |
|---|---|
| The presence of particle adds some complexity to the thermal behavior of the polymeric matrix. One of the most common effects is that the silica particle could serve as a nucleating agent, thus providing a large number of nucleation sites and allowing the polymer to crystallize at higher temperatures. This contributes to a change in the morphology of the system and in some cases, depending on the processing condition, a phase change occurs. | The presence of clay adds some complexity to the crystallization behavior of the polymeric matrix. One of the most common observations is that the clay could serve as a nucleating agent, thus providing a large number of nucleation sites and allowing the polymer to crystallize at higher temperatures. This contributes to a change in the morphology of the system, usually reflected in a larger number of smaller crystallites per unit volume. In some cases, depending on processing conditions, a phase change occurs. |
|
|
| [66.] Mrs/Fragment 102 03 - Diskussion Bearbeitet: 18. May 2015, 20:38 WiseWoman Erstellt: 25. January 2015, 10:01 (Klgn) | Bhattacharya et al. 2008, Fragment, Gesichtet, Mrs, SMWFragment, Schutzlevel sysop, Verschleierung |
|
|
| Untersuchte Arbeit: Seite: 102, Zeilen: 3-8 |
Quelle: Bhattacharya et al. 2008 Seite(n): 109; 110, Zeilen: 109: 12 ff.; 110: 16-20 |
|---|---|
| Crystallization kinetics and the morphology of crystallized products are strongly influenced by cooling rate, system pressure and the presence of particles. Melting and crystallization of crystalline polymers generally occurs over a range of temperature, because of the presence of a distribution of molecular weights and the mixed crystalline/amorphous phase in the sample. Polymer crystals occur in a variety of crystallite sizes and phases (e.g. α, β, γ, etc.). These phases may exhibit different melting/crystallization temperature. | [S. 109]
Crystallization kinetics and the morphology of crystallized products are strongly influenced by cooling rate, system pressure and the presence of clay. [S. 110] Melting and crystallization of crystalline polymers generally occur over a range of temperatures, because of the presence of a distribution of molecular weights and the mixed crystalline/amorphous phases in the sample. Polymer crystals occur in a variety of crystallite sizes and phases (e.g., α, β, γ, etc.). These phases may exhibit different melting/crystallization temperatures. |
The source is given on page 103 for the figure. |
|
| [67.] Mrs/Fragment 119 23 - Diskussion Bearbeitet: 16. May 2015, 19:35 Hindemith Erstellt: 24. March 2015, 13:57 (SleepyHollow02) | Fragment, Gesichtet, Mrs, Nairn 2007, SMWFragment, Schutzlevel sysop, Verschleierung |
|
|
| Untersuchte Arbeit: Seite: 119, Zeilen: 23-28 |
Quelle: Nairn 2007 Seite(n): 7, Zeilen: 4ff |
|---|---|
| And for examples many influence on mechanical properties such as stiffness, strength, viscoelasticity, toughness, and viscosity. If molecular weight is too low, the transition temperatures and the mechanical properties will generally be too low for the polymer material to have any useful commercial applications. For a polymer to be useful it must have transition temperatures above room temperatures and it must have mechanical properties sufficient to bear design loads. | Some examples include the temperatures for transitions from liquids to waxes to rubbers to solids and mechanical properties such as stiffness, strength, viscoelasticity, toughness, and viscosity. If molecular weight is too low, the transition temperatures and the mechanical properties will generally be too low for the polymer material to have any useful commercial applications. For a polymer to be useful it must have transition temperatures to waxes or liquids that are above room temperature (i.e., be a solid at room temperature) and it must have mechanical properties sufficient to bear design loads. |
The source is not mentioned. The passage can also be found in [4], a source from 2003 (PDF file properties), which, however, is difficult to cite. The same passage has been used also in Mrs/Fragment 067 05. The beginning of the sentence is grammatically incorrect, it should be "And for example many influences on ...", but the sentence is not completed. |
|
| [68.] Mrs/Fragment 125 06 - Diskussion Bearbeitet: 16. May 2015, 11:18 Hindemith Erstellt: 23. March 2015, 11:08 (SleepyHollow02) | Fragment, Gesichtet, Mrs, SMWFragment, Schutzlevel sysop, Verschleierung, Yang et al 2007 |
|
|
| Untersuchte Arbeit: Seite: 125, Zeilen: 6-8 |
Quelle: Yang et al 2007 Seite(n): 438, Zeilen: l.col. 31 ff. - r. col 3 |
|---|---|
| Similarly, the melting enthalpy decreased significantly by the presence of nanoparticle. This shift can also be attributed to the fact that the polyamide chains are anchored to the silica surface thus reducing the PA6 chains mobility. | Similarly, the melting enthalpy decreased significantly by the presence of nanosilica. This shift can also be attributed to the fact that the polyamide chains are anchored to the silica surface thus reducing the PA6 chains mobility. |
The copied text is short, but it describes an insight that apparently is not from the author of the thesis discussed. |
|
| [69.] Mrs/Fragment 132 07 - Diskussion Bearbeitet: 19. April 2015, 18:57 WiseWoman Erstellt: 28. December 2014, 01:10 (Hindemith) | Fragment, Gesichtet, Mahfuz et al 2008, Mrs, SMWFragment, Schutzlevel sysop, Verschleierung |
|
|
| Untersuchte Arbeit: Seite: 132, Zeilen: 7-10, 11-18 |
Quelle: Mahfuz et al 2008 Seite(n): 6, Zeilen: 6. 38 ff. |
|---|---|
| Three basic bonds of polyamide 6, i.e. amide N–H at 3297 cm-1, hydroxyl O–H at 2800–3000 cm-1, and carbonyl C=O at 1637 cm-1 were of primary interest. It is seen in Figure 8.11(b) that IR absorbance for each of the three basic bonds has increased significantly, characterized by their sharper and higher peaks. Higher peak and larger area under the curve corresponds to higher absorption of light energy of the chemical bond concerned. In other words, the IR absorbance is a direct measure of the interaction with the molecular environment, indicating that SiO2 reinforcement into polyamide was responsible for such an increase. On the other hand, after functionalization of SiO2 particles, i.e. in Figure 8.11(c), it is seen that the wave number of the three basic bond are maintained, while in addition, a siloxane Si–O–Si bond at 1090 cm-1 is formed which was not seen with Figures 8.11(a). This is what was expected from functionalization; establishing a continuous covalent linkage across the particle (silica) and polymer (polyamide 6) interface [107].
[107] H. Mahfuz, M. Hasan, V. Dhanak, Nanotechnology 19 (2008) 445702 (7pp) |
Three basic bonds of nylon 6, i.e., amide N-H @ 3297 cm-1, hydroxyl O-H @ 2800-3000 cm-1, and carbonyl C=O @ 1637 cm-1 were of primary interests. It is seen in Fig 8.b that IR absorbance for each of the three basic bonds has increased significantly characterized by their sharper and higher peaks. The IR absorbance is a direct measure of bond strength indicating that SiO2 reinforcement into nylon was responsible for such increase. On the other hand after functionalization of SiO2 particles, i.e., in
[page 7] Fig 8.c it is seen that three basic bond strengths are maintained, while in addition, a siloxane Si-O-Si bond @1090 cm-1 is formed which was not seen with Fig 8.a or 8.b.This is what we expected from functionalization; establishing a continuous covalent linkage across the particle (silica) and polymer (Nylon 6) interface. |
The source is not mentioned. Possibly the passage can also be found in reference [107] (to be checked), but even if this was the case, it would not become clear that the whole documented passage is taken from the source. In particular, it does not become clear that what is described are not experiments of M . R. S., but of other authors. |
|
| [70.] Mrs/Fragment 133 01 - Diskussion Bearbeitet: 19. April 2015, 19:02 WiseWoman Erstellt: 28. December 2014, 01:24 (Hindemith) | Fragment, Gesichtet, Mahfuz et al 2008, Mrs, SMWFragment, Schutzlevel sysop, Verschleierung |
|
|
| Untersuchte Arbeit: Seite: 133, Zeilen: figure |
Quelle: Mahfuz et al 2008 Seite(n): 7, Zeilen: figure |
|---|---|
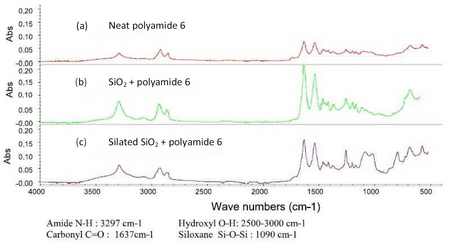
Figure 8.11 FTIR spectra (a) pure PA6 fiber, (b) PA6 fiber with 0.5% silica, (c) PA6 fiber with 0.5 % modified silica. |

Fig 8: FTIR Spectroscopy – (a) neat nylon 6, (b) nylon 6 with 1wt% silica, and (c) nylon 6 with 1 wt% functionalized silica. |
The source is not mentioned here. The reader gets the wrong impression that he is presented with measurements of M. R. S.. Note that on the previous page 132, line 3, one reads:
Note also the caption: M. R. S. claims that slightly different fibers have been analysed in the dissertation as compared to the source. Compare also the text before this figure on the previous page, which has also been taken from the source (and where the source is mentioned): Fragment 132 07 Close-ups of the green curve hitting the x-axis above: Note:
|
|
| [71.] Mrs/Fragment 134 01 - Diskussion Bearbeitet: 22. March 2015, 13:41 WiseWoman Erstellt: 27. December 2014, 15:29 (Hindemith) | Fornes 2003, Fragment, Gesichtet, Mrs, SMWFragment, Schutzlevel sysop, Verschleierung |
|
|
| Untersuchte Arbeit: Seite: 134, Zeilen: 1-10 (complete) |
Quelle: Fornes 2003 Seite(n): 265, Zeilen: 3ff |
|---|---|
| Non-Isothermal crystallization of polyamide 6 nanocomposites fibers
An extensive analysis of non-isothermal crystallization behavior was carried out on pure polyamide 6 and its nanocomposites. To rightfully compare pure polyamide 6 materials with its nanocomposites, it was necessary to extrude the pure polyamide under the same processing conditions used to form the nanocomposites. Regardless of the molecular weight, the extruded materials exhibit faster crystallization than the virgin samples (Figure 8.12 labeled as (1)). There are several possible reasons why such behavior is observed. Faster crystallization may arise from increased nucleation due to the presence of impurities incorporated in the matrix during processing. Memory effects associated with thermal and stress histories that remain present in the sample after annealing in the melt may also lead to an increased rate of crystallization. |
Isothermal Crystallization of Nylon 6 Nanocomposites
An extensive analysis of isothermal crystallization behavior was carried out on pure nylon 6 and its nanocomposites. To rightfully compare pure nylon 6 materials with its nanocomposites, it was necessary to extrude the pure polyamides under the same processing conditions used to form the nanocomposites. Figure 9.4 shows the isothermal crystallization behavior of virgin, i.e., from as-received pellets, and extruded nylon 6 materials. Regardless of the molecular weight, the extruded materials exhibit faster crystallization than the virgin samples. There are several possible reasons why such behavior is observed. Faster crystallization may arise from increased nucleation due to the presence of impurities incorporated in the matrix during processing. Memory effects associated with thermal and stress histories that remain present in the sample after annealing in the melt may also lead to an increased rate of crystallization. |
A source is not mentioned. The description from Fornes' thesis is also to be found at Fornes, T.D. & Paul, D. R. (2003). Crystallization behavior of nylon 6 nanocomposites. In Polymer 44, pp. 3945–3961. The figures referred to are not the same. |
|
| [72.] Mrs/Fragment 135 01 - Diskussion Bearbeitet: 22. March 2015, 13:40 WiseWoman Erstellt: 27. December 2014, 15:56 (Hindemith) | BauernOpfer, Fornes 2003, Fragment, Gesichtet, Mrs, SMWFragment, Schutzlevel sysop |
|
|
| Untersuchte Arbeit: Seite: 135, Zeilen: 1-3, 4-14 (complete, without figure) |
Quelle: Fornes 2003 Seite(n): 265, 266, 267, Zeilen: 265: 12ff; 266: 1ff; 267: 5ff |
|---|---|
| Numerous studies have shown that the processing history of the polymer, e.g. melting, mixing, cooling, pelletizing, etc. is often not fully erased when the polymer is annealed at high melt temperatures [289,–320-325].
[Figure 8.12] Figure 8.12 Crystalline temperature of virgin PA6 (1) and pure extruded PA6 (2). Faster crystallization may arise from increased nucleation due to the presence of impurities incorporated in the matrix during processing. Aharoni [173] reports that any shearing imposed on the polymer during melt processing may facilitate the production of aligned arrays of bridged H-bonded sections that act as stable nuclei, capable of remaining intact in the melt state for long periods of time. These arrays ultimately act as crystallization sites upon cooling from the melt. Indeed, Khanna et al. [321] showed that extruded polyamide 6 leads to faster crystallization than virgin polyamide 6 under both isothermal and non-isothermal conditions. The temperature of melting and the length of time held in the molten state will also determine the amount of stable nuclei remaining prior to recrystallization; this issue has been the topic of many investigations [325-330]. The above demonstrates that processing alone significantly affects the crystallization behavior of polyamide 6; therefore, for proper comparison between pure polyamide 6 and the nanocomposites, the 0 wt. % SiO2 data presented in the remaining plots represent the extruded version of polyamide 6. [173] S.M: Aharoni, n-Polyamides, their synthesis, structure, and properties. Chichester; New York: Wiley; 1997. [289] T.D.Fornes, D.R.Paul, Polymer 44 (2003) pp. 3945-3961 [320] Y.P. Khanna, R. Kumar, A.C. Reimschuessel, Polym Eng Sci 1988; 28(24):1607–11. [321] Y.P. Khanna, A.C. Reimschuessel, A. Banerjie, Polym Eng Sci 1988; 28(24):1600–6. [322] Y.P. Khanna, R. Kumar, A.C. Reimschuessel, Polym Eng Sci 1988; 28(24):1612–5. [323] Y.P. Khanna, A.C. Reimschuessel, J Appl Polym Sci 1988; 35(8): 2259–68. [324] Y.P. Khanna, Polym Eng Sci 1990; 30(24):1615–9. [325] N. Avramova, Polym Polym Compos 1993; 1(4):261–74. [326] JH. Magill, Polymer 1962; 3(No. 1):43–51. [327] E. Turska, S. Gogolewski, Polymer 1971;12(10):616–28. [328] E. Turska, S. Goglewski, J Appl Polym Sci 1975;19(3):637–44. [329] N. Avramova, S. Fakirov, I. Avramov, J Polym Sci, Polym Phys 1984; 22(2):311–3. [330] N. Avramova, S. Fakirov, J Polym Sci, Polym Phys 1986; 24(4):761–8. |
Faster crystallization may arise from increased nucleation due to the presence of impurities incorporated in the matrix during processing. [...] Numerous studies have shown that the processing history of the polymer, e.g., melting, mixing, cooling, pelletizing, etc., are often not fully erased when the polymer is annealed at high melt temperatures [6, 58-63]. Aharoni reports that any shearing imposed on the polymer during melt processing may facilitate the production of aligned arrays of H-bonded sections that act as stable
[page 266] nuclei, capable of remaining intact in the melt state for long periods of time [6]. These arrays ultimately act as crystallization sites upon cooling from the melt. Indeed, Khanna et al. showed that extruded nylon 6 leads to faster crystallization than virgin nylon 6 under both isothermal and non-isothermal conditions [59]. The temperature of melting and the length of time held in the molten state will also determine the amount of stable nuclei remaining prior to recrystallization; this issue has been the topic of many investigations [63-68]. [page 267] The above demonstrates that processing alone significantly affects the crystallization behavior of nylon 6; therefore, for proper comparison between pure nylon 6 and the nanocomposites, the 0 wt% MMT data presented in the remaining plots represent the extruded version of nylon 6. 6. Aharoni, SM, n-Nylons, their synthesis, structure, and properties. New York: J. Wiley & Sons. 1997. 58. Khanna, YP, Kumar, R, Reimschuessel, AC. Polym Engng Sci 1988;28(24): 1607-11. 59. Khanna, YP, Reimschuessel, AC, Banerjie, A, Altman, C. Polym Engng Sci 1988;28(24): 1600-6. 60. Khanna, YP, Kumar, R, Reimschuessel, AC. Polym Eng Sci 1988;28(24): 1612-5. 61. Khanna, YP, Reimschuessel, AC. J Appl Polym Sci 1988;35(8): 2259-68. 62. Khanna, YP. Polym Engng Sci 1990;30(24): 1615-9. 63. Avramova, N. Polymers & Polymer Composites 1993;1(4): 261-74. 64. Magill, JH. Polymer 1962;3(No. 1): 43-51. 65. Turska, E, Gogolewski, S. Polymer 1971;12(10): 616-28. 66. Turska, E, Goglewski, S. J Appl Polym Sci 1975;19(3): 637-44. 67. Avramova, N, Fakirov, S, Avramov, I. J Polym Sci, Part B 1984;22(2): 311-3. 68. Avramova, N, Fakirov, S. Journal of Polymer Science, Part B 1986;24(4): 761-8. |
A source is not mentioned. The description from Fornes' thesis is also to be found at Fornes, T.D. & Paul, D. R. (2003). Crystallization behavior of nylon 6 nanocomposites. In Polymer 44, pp. 3945–3961. The figures referred to are not the same. This reference is given as number 289 by Mrs and referenced here, but it would only cover the sentence before the reference, not the entire page including all referenced work. Thus, this fragment is marked as a "BauernOpfer" (pawn sacrifice). |
|
| [73.] Mrs/Fragment 145 09 - Diskussion Bearbeitet: 25. April 2015, 22:28 WiseWoman Erstellt: 28. December 2014, 23:18 (Hindemith) | Fragment, Gesichtet, Mrs, SMWFragment, Schutzlevel sysop, Van Zyl et al 2002, Verschleierung |
|
|
| Untersuchte Arbeit: Seite: 145, Zeilen: 9-11 |
Quelle: Van Zyl et al 2002 Seite(n): 109, Zeilen: l.col: 18 ff. |
|---|---|
| The silica-water system presumably led to more aggregation and probably some degree of degradation, while in the case of silica-ethanol system, these obstacles were either minimized or not encountered. | The water vs. EtOH difference was ascribed to the media's different behavior upon composite melting during moulding, in which case the silica-water system presumably led to more aggregation and probably some degree of degradation, while in the case of silica-ethanol system, these obstacles were either minimized or not encountered. |
The source is not mentioned. See also Mrs/Fragment 148 20 |
|
| [74.] Mrs/Fragment 148 20 - Diskussion Bearbeitet: 25. April 2015, 22:27 WiseWoman Erstellt: 28. December 2014, 23:17 (Hindemith) | Fragment, Gesichtet, Mrs, SMWFragment, Schutzlevel sysop, Van Zyl et al 2002, Verschleierung |
|
|
| Untersuchte Arbeit: Seite: 148, Zeilen: 20-22 |
Quelle: Van Zyl et al 2002 Seite(n): 109, Zeilen: l.col: 18 ff. |
|---|---|
| Using different dispersion media in in-situ polymerization techniques shows that the silica-water system presumably led to more aggregation and probably some degree of degradation, while in the case of silica-ethanol system, these obstacles were either minimized or not encountered. | The water vs. EtOH difference was ascribed to the media's different behavior upon composite melting during moulding, in which case the silica-water system presumably led to more aggregation and probably some degree of degradation, while in the case of silica-ethanol system, these obstacles were either minimized or not encountered. |
The source is not mentioned. See also: Mrs/Fragment 145 09 |
|





















